2024 Predictions for Soft-Tech Product, Systems, and Software Development
As the SoftTech sector moves into 2024, we aim to gain a deeper insight into the factors driving transformation in the development of products, systems, and software and explore how teams within this industry are adapting to meet the challenges posed by these evolving complexities.
In part five of this six-part series, we asked our own industry experts Patrick Garman – Principal Solutions Consultant, and Steven Meadows – Principal Solutions Lead, to weigh in on the SoftTech trends they’re anticipating in the coming year and beyond.
We like to stay on top of trends in other industries as well. Read our Automotive predictions HERE, Aerospace & Defense HERE, Industrial & Consumer Electronics (ICE) HERE, Medical Device & Life Sciences HERE, and Product & Engineering Teams HERE.
Design Trends – What are the biggest trends you’re seeing in your industry right now? How will they impact SoftTech development?
Patrick Garman: With the increased awareness and popularity of tools like ChatGPT, Generative AI and its potential applications in product development and requirements management has come up often in conversations with customers. Common questions include: can AI suggest ‘missing’ requirements, suggest relationship links for existing requirements, or even generate a full set of requirements based on similar products or projects? These are interesting questions, and I can certainly see the potential value that AI could add, but softtech companies should be wary of automating too much of their requirements management and product development processes. Generative AI might be able to provide suggestions or at least a starting point for requirements, but it cannot and should not replace human review and insights.
Steven Meadows: Artificial Intelligence (AI) is evolving at an unprecedented rate and the continuation of the application of AI in software development is no exception. More tools and libraries are being built to help support the automation of development tasks, including coding and test automation. Developers are now able to create more intelligent and user-centric systems, which ultimately improves the stakeholder experience. Over the next few years, I anticipate that we’ll start to see more AI-based applications that will help teams debug code and fix bugs in real-time.
In terms of requirements management in the software world, we’re seeing AI and machine learning assist with tasks such as requirement generation. This will continue to help shape better-quality systems with fewer issues for users. Models in this area are still being improved, but we are already seeing the benefits of AI bringing better-quality systems to market.
Regulations – What changing regulatory guidelines do you anticipate having an impact on companies in 2024?
Garman: Data privacy still looms large in SoftTech and is top of mind for consumers. A 2020 Pew Research study indicated that half of U.S. consumers have not used a product because of privacy concerns. While the European Union acted several years ago by introducing GDRP, the United States has shied away from federal regulation – focusing on specific classes of data (health and financial) and users (children). Traction for data privacy regulation at the state level is gaining speed, though. California introduced the California Privacy Rights Act in 2019, laws are being enacted in Virginia, Colorado, Utah, and Connecticut this year, and eight more states have signed data privacy legislation, with another six currently debating the issue. With the continued growth of connected devices and cloud services, and the emergence of advanced AI, I predict even more attention will be directed to how tech companies collect and use consumer data. To prepare, forward-looking tech companies will take a cue from companies like Apple and take a more proactive ‘self-regulating’ approach to data privacy in their product design.
Meadows: Keeping with the theme of AI, I anticipate that there will be new regulations coming out affecting the application and use of AI in software. Governments and state entities have made it clear that further regulation of AI is coming, so it’s only a matter of time.
It’s clear to see that there have been instances of biased results being produced by AI systems. For example, credit card algorithms that discriminate based on sex and other factors. Algorithms have also been developed that target people based on race, religion, and gender.
It’s unclear if there will be mandates on the particular use of training data as part of a model’s learning phase or whether limitations will be placed on the types of models used. One thing is for certain though – further regulation of AI in software is coming!
Tool Innovation – From a SoftTech industry engineering toolset perspective, what are some of the processes you think forward-thinking firms will be working to leverage or incorporate into their process, and why?
Garman: While it might seem like a step back for some, I think there is a strong movement in software development to find a better balance between planning and implementation activities. Many companies take Agile to mean ‘just go do’ — start writing code as quickly as possible, release to customers early and often, learn lessons on the fly, and incorporate feedback rapidly. That’s certainly part of Agile and a great ideal to strive for, but in practice, it is very difficult to incorporate customer feedback into iterative releases quickly enough. In other words, it’s easy to ‘fail fast’ but very hard to ‘course correct fast.’ That’s not the fault of software teams; there is a lot of pressure to deliver an ever-growing backlog of features and stories, and prioritization is difficult to manage when business objectives and market demands can change overnight! Applying more diligence to the planning activities – defining and getting agreement on requirements before ‘just going and doing’ – goes a long way towards improving software teams’ ability to actively prioritize their backlogs, feel confident that what they are doing is the right thing to be doing, and reduces the amount of rework or technical debt that must be addressed post-release. Adopting a requirements management tool that supports an agile approach will add tremendous value in SoftTech development.
Meadows: Software companies continue to adopt, and rightly so, an Agile work culture and methodology. Forward-thinking Agile teams must be prepared to adapt to challenges that can hinder quality development for their customers. Task management tools like Jira and Azure DevOps (ADO) have become standard ways to manage work including the prioritization of activities, project management, and resource allocation. One aspect of development that is very much neglected is requirements management. Development teams need to be able to effectively communicate with business analysts, product owners, architects, and their own customers, as well as understand whether requirements have been satisfied in real time.
Without a dedicated and purpose-built requirements management system, silos are created in terms of data and teams, leading to systems produced with more defects and lower quality. Forward-thinking- teams should be adopting a requirements tool tightly coupled with their task management applications for effective end-to-end visibility throughout the development cycle, catching issues and mitigating risk earlier in the development lifecycle.
RELATED: Buyer’s Guide: Selecting a Requirements Management and Traceability Solution for Software Development
What role will cybersecurity play in soft-tech industry development in the coming year and beyond?
Garman: Cybersecurity is perhaps the most important consideration for product development in SoftTech. The convenience of connected devices will continue to drive consumer demand – even my dishwasher connects to the internet! However, this convenience comes with the risk of data breaches and network vulnerabilities. Encrypting data during transmission and storage is just table stakes now. SoftTech companies must be ready to move much faster than in the past to push software and firmware updates in response to new vulnerabilities. The ability to quickly generate impact analyses and trace- identified risks and vulnerabilities to the mitigating requirements is more important than ever in taking a proactive stance toward cybersecurity.
In your opinion, what are the biggest differences between SoftTech companies that will survive to see 2030, and ones that don’t?
Garman: Technical debt is becoming a much larger liability for SoftTech companies as the rate of innovation continues to accelerate – the more technical debt a SoftTech company builds, the harder it will become to quickly respond to emerging trends and innovations. We see it more and more often – once the better mouse trap is available, it becomes the expectation, not the nice to have. Of course, decisions must be made to address near-term or immediate needs and there will always be trade-offs to consider to optimize ROI. SoftTech companies that design and develop their products to not only to fulfill the near-term needs while maintaining the architectural flexibility to adopt future trends will be the ones to keep pace with consumer expectations and win in the long term.
What advice would you give to new companies entering the SoftTech industry?
Garman: Embrace design thinking and avoid jumping too quickly to a solution. This applies if you are a new company or an established company entering a new market. SoftTech products can become commodities very quickly – it’s easier than ever to just copy/paste an existing solution – but that will ultimately only drive prices down as more options are available to consumers. Design thinking is a great framework for requirements management. Start by really defining the problem or needs that your product intends to resolve and also defining the user needs for your target market. User needs are the foundation for good requirements, and good requirements are the foundation for successful products.
What topic(s) do you wish companies were paying more attention to?
Garman: Refocus on user experience. MVP is commonly defined as “Minimum Viable Product,” but I strongly prefer “Minimum Valuable Product”– in other words, instead of designing through the lens of ‘what is the least we can deliver so that a user can accomplish this task or goal,’ adopt a mindset of ‘what is the least we can deliver so that a user has a good experience in accomplishing this task or goal.’ Designing for good user experience does not limit the return on investment (ROI) – in fact, it leads to higher lifetime value through customer loyalty and goodwill.
What is the biggest mistake you see companies in the soft-tech industry making right now?
Garman: I mentioned earlier that I see the trend of software teams re-prioritizing requirements management and planning activities in advance of development activities, and that is in direct response to the issues and pain points that SoftTech companies have experienced as they adopt a ‘just go do’ approach to product development. One of my grad school professors claimed that an 80/20 ratio of planning to doing was the ideal. Every company will need to find its own balance, but the data is clear – companies that apply diligence in requirements management are faster to market, expend less time and resources in the actual development phase, and experience fewer defects after release.
RELATED: Traceable Agile – Speed AND Quality Are Possible for Software Factories in Safety-critical Industries
Do you think there will be any major disruptors in the SoftTech industry in the coming year? How do you think it will impact the industry?
Garman: At the risk of sounding like a broken record, advancements in artificial intelligence. The potential applications are tremendous! I’ve mentioned the potential for using AI to develop products. In the short term, we’ll likely see more soft-tech companies employing generative AI for product support and predictive process automation. Conversational AI will also change the way we interact with software and connected devices. The market for voice assistants has a projected CAGR of nearly 27% over the next eight years and hands-free devices are projected to have a 7% CAGR over the next five years – and that is based on the current task-based commands that are supported. As consumers continue to adopt smart home devices, the expectations for hands-free control will only increase.
What do you predict for regulation in the SoftTech industry in 2024?
Will those trends still be prevalent five years from now? 10 years?
Garman: I’ve already discussed data privacy regulation, and I do think that the federal regulations will be expanded in the United States in the coming years. Regulation for AI – specifically generative AI – is likely next, but what will be enacted and how is still an open question. The two topics are linked in that generative AI produces content based on existing inputs, generally user data and public-facing IP. AI and advanced machine learning have tremendous potential, but aside from data privacy concerns, AI also introduces safety risks. We are already seeing the implementation of functional safety standards in robotics, and as autonomous robots continue to advance, we will likely see increased regulatory oversight. No one wants the rise of Skynet!



![[Webinar Recap] Bridging the Gap in Insurance Product Development [Webinar Recap] Bridging the Gap in Insurance Product Development](https://www.jamasoftware.com/media/2023/10/Bridging-the-Gap-in-Insurance-Product-Development.png)


![[Webinar Recap] Manage by Exception: Data-driven Practices to Improve Product Quality [Webinar Recap] Manage by Exception: Data-driven Practices to Improve Product Quality](https://www.jamasoftware.com/media/2023/08/2023-Webinar-Recap-Med-Update-4.png)
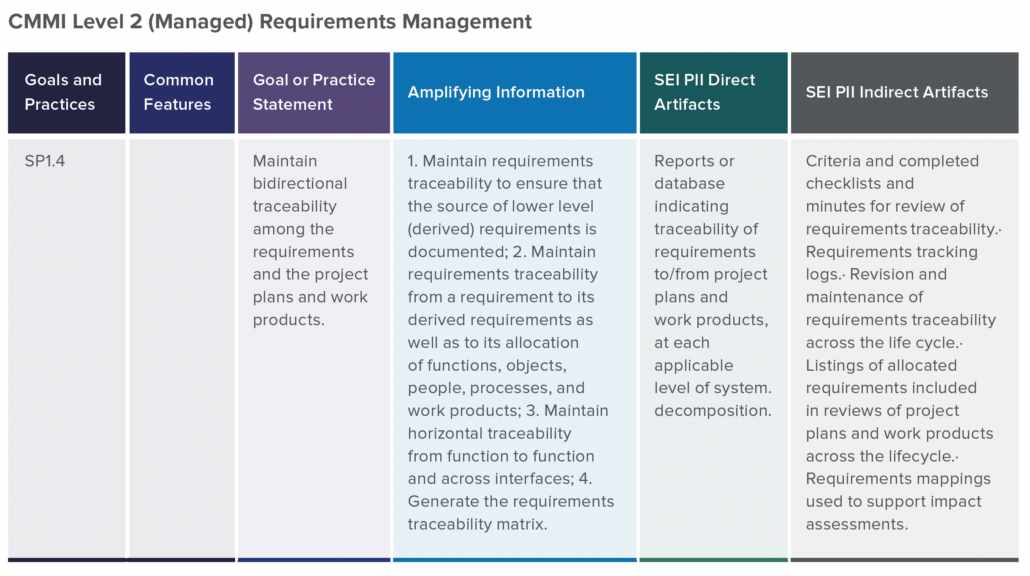
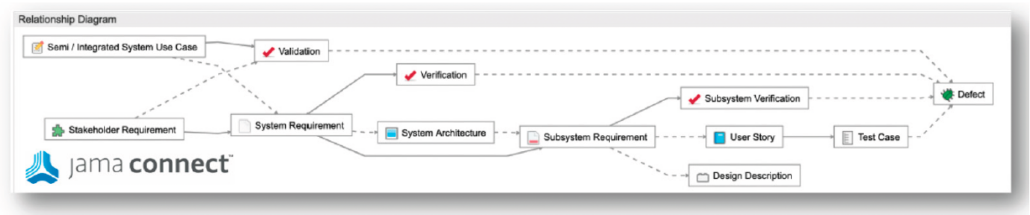
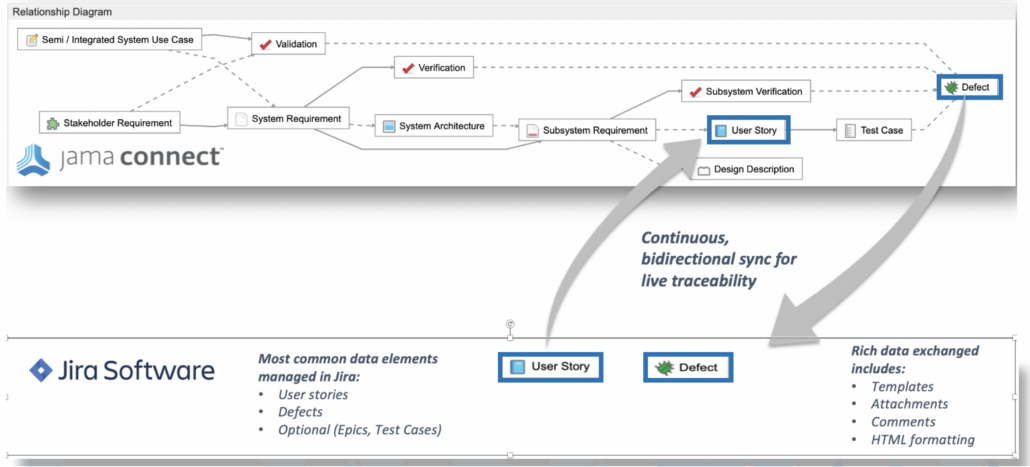
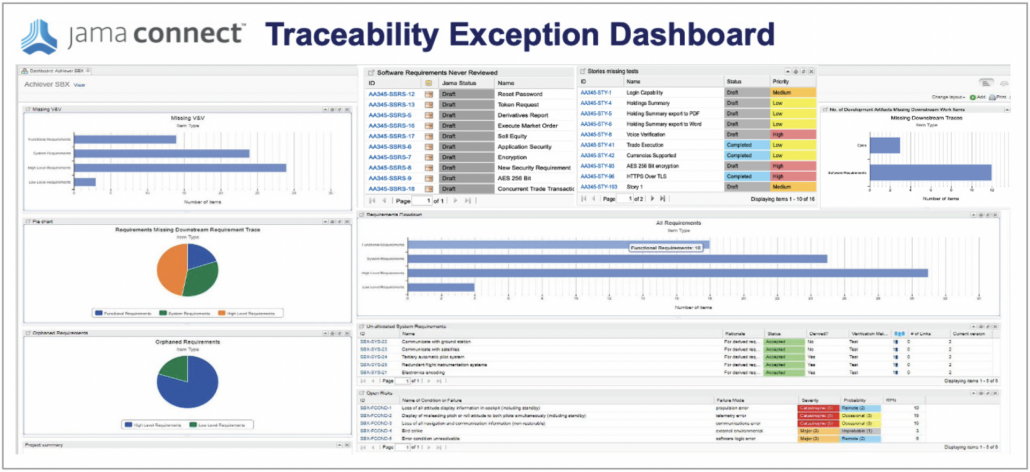
 In part two of this two-part blog series, we continue the overview of our recent whitepaper, “How to Achieve Higher Levels of the Capability Maturity Model Integration (CMMI) with Live Traceability™” Click
In part two of this two-part blog series, we continue the overview of our recent whitepaper, “How to Achieve Higher Levels of the Capability Maturity Model Integration (CMMI) with Live Traceability™” Click 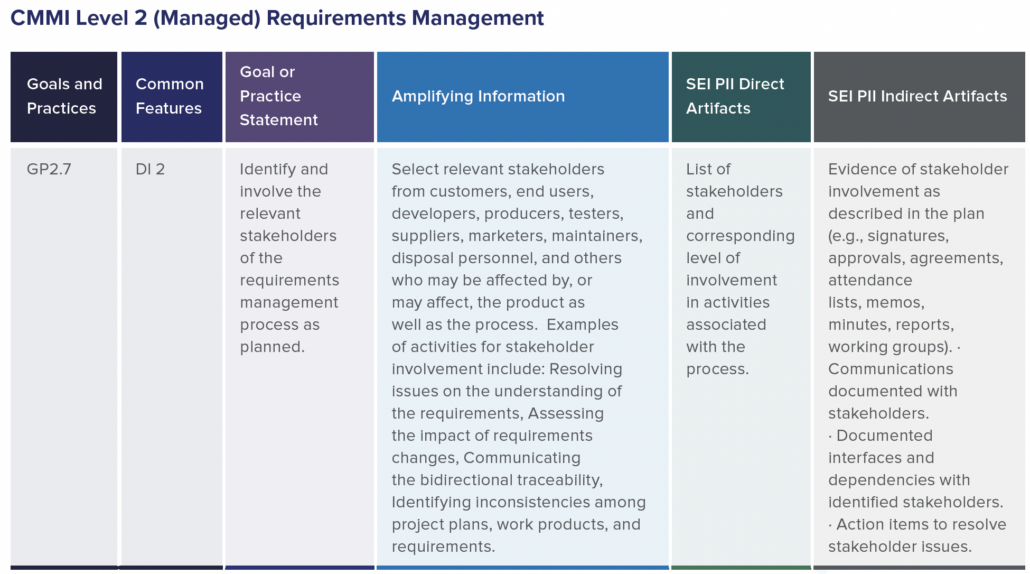
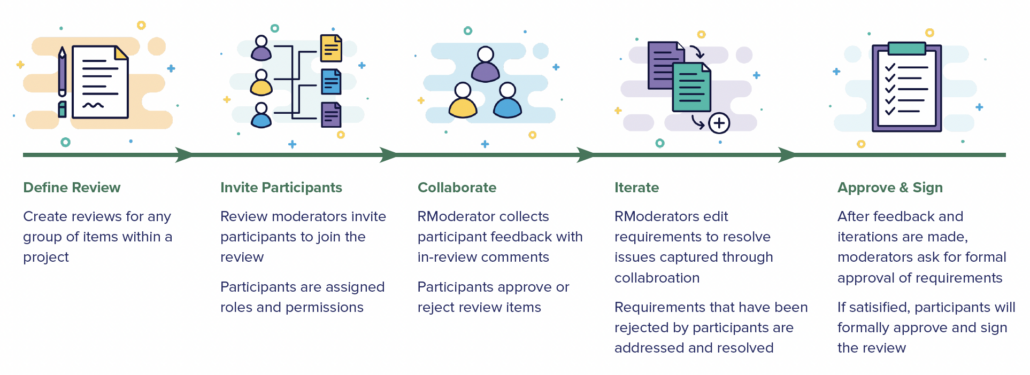
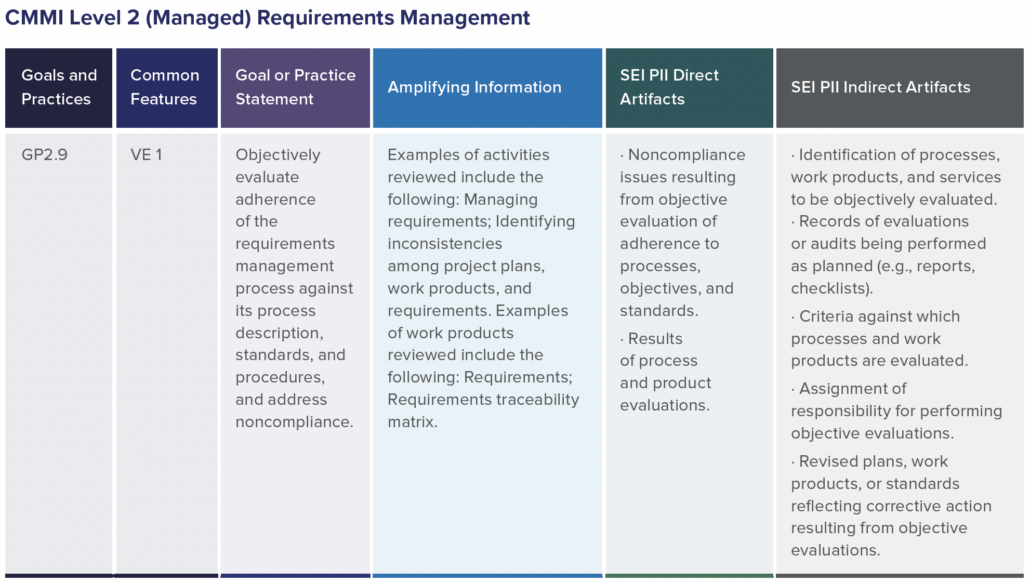
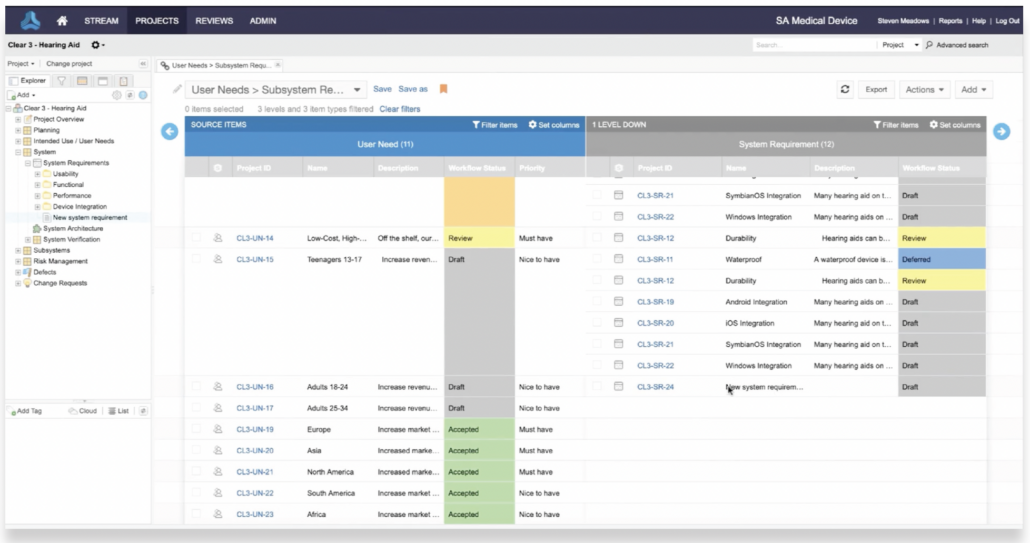
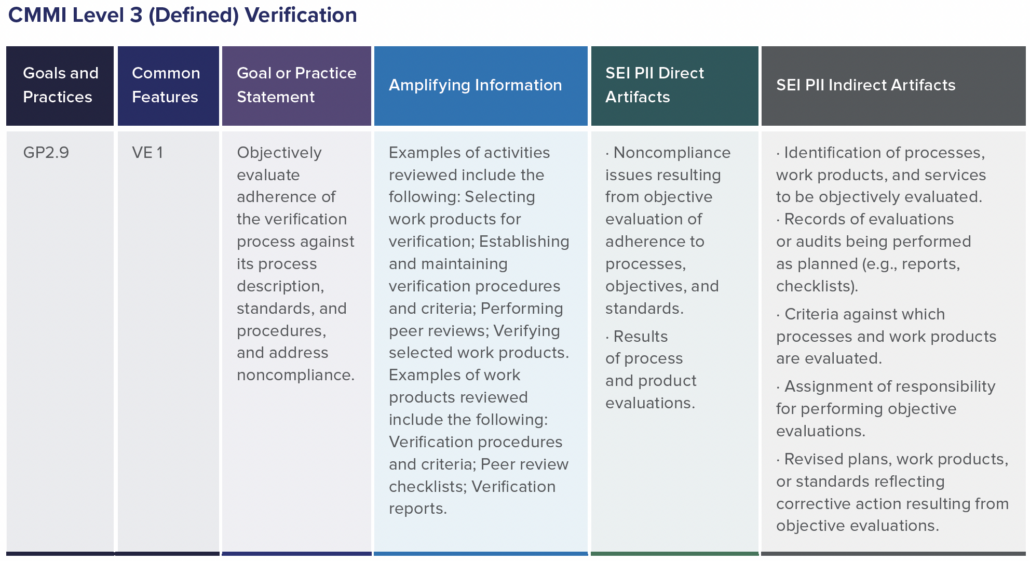
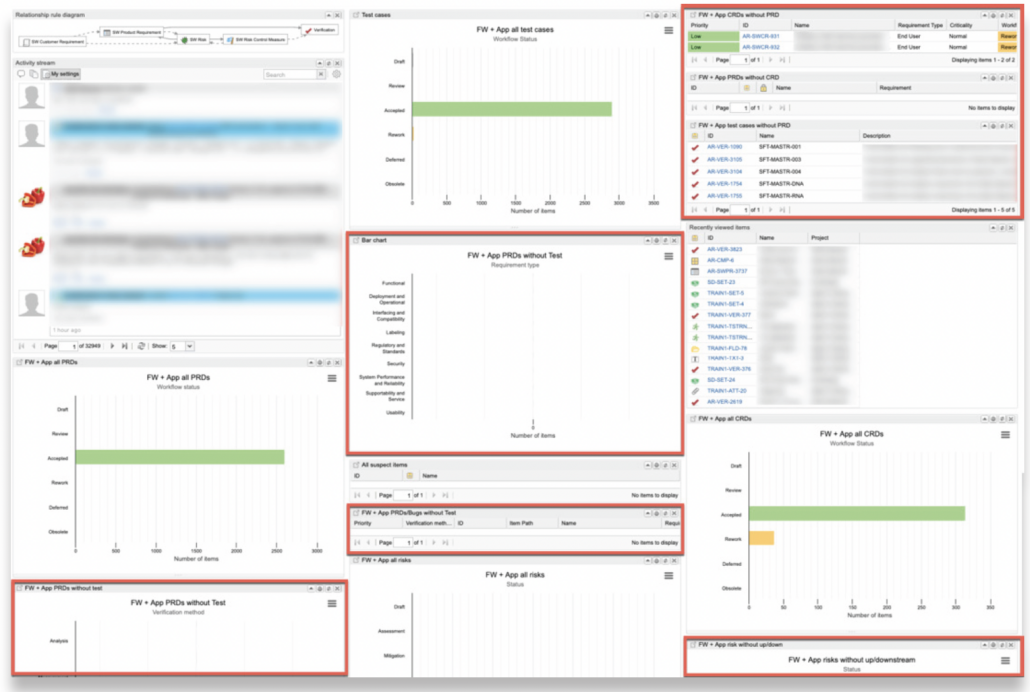
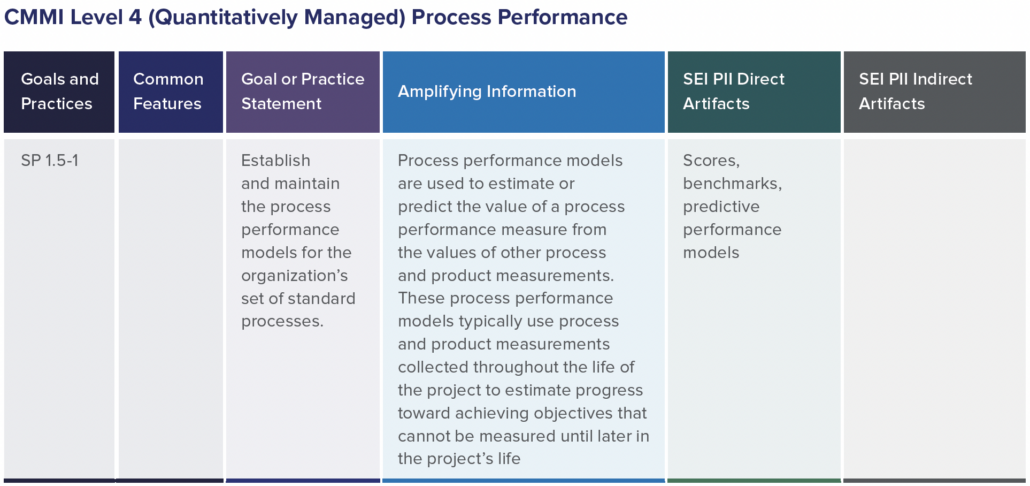
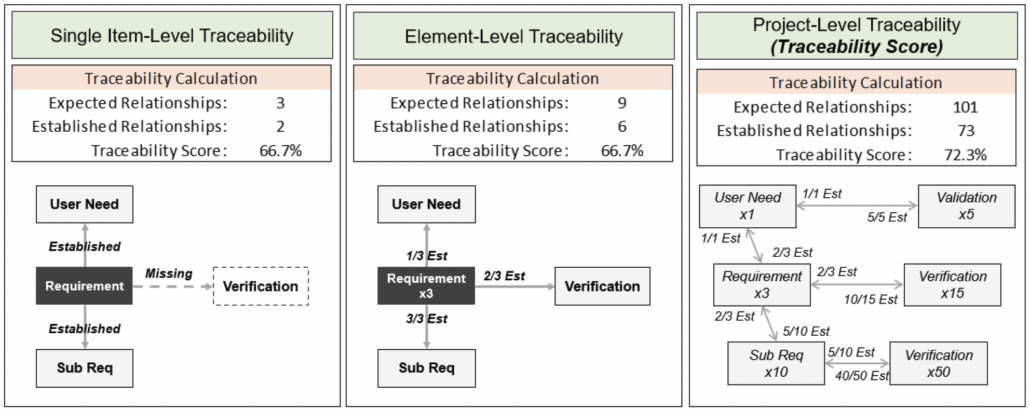
 In part one of this two-part blog series, we provide an overview of our recent whitepaper, “How to Achieve Higher Levels of the Capability Maturity Model Integration (CMMI) with Live Traceability™” Click
In part one of this two-part blog series, we provide an overview of our recent whitepaper, “How to Achieve Higher Levels of the Capability Maturity Model Integration (CMMI) with Live Traceability™” Click