What is Requirements Traceability? Importance Explained
The Essential Guide to Requirements Management and Traceability
Chapters
- 1. Requirements Management
- Overview
- 1 What is Requirements Management?
- 2 Why do you need Requirements Management?
- 3 Four Stages of Requirements Management Processes
- 4 Adopting an Agile Approach to Requirements Management
- 5 Status Request Changes
- 6 Conquering the 5 Biggest Challenges of Requirements Management
- 7 Three Reasons You Need a Requirements Management Solution
- 8 Guide to Poor Requirements: Identify Causes, Repercussions, and How to Fix Them
- 2. Writing Requirements
- Overview
- 1 Functional requirements examples and templates
- 2 Identifying and Measuring Requirements Quality
- 3 How to write system requirement specification (SRS) documents
- 4 The Fundamentals of Business Requirements: Examples of Business Requirements and the Importance of Excellence
- 5 Adopting the EARS Notation to Improve Requirements Engineering
- 6 Jama Connect Advisor™
- 7 Frequently Asked Questions about the EARS Notation and Jama Connect Advisor™
- 8 How to Write an Effective Product Requirements Document (PRD)
- 9 Functional vs. Non-Functional Requirements
- 10 What Are Nonfunctional Requirements and How Do They Impact Product Development?
- 11 Characteristics of Effective Software Requirements and Software Requirements Specifications (SRS)
- 12 8 Do’s and Don’ts for Writing Requirements
- 3. Requirements Gathering and Management Processes
- Overview
- 1 Requirements Engineering
- 2 Requirements Analysis
- 3 A Guide to Requirements Elicitation for Product Teams
- 4 Requirements Gathering Techniques for Agile Product Teams
- 5 What is Requirements Gathering in Software Engineering?
- 6 Defining and Implementing a Requirements Baseline
- 7 Managing Project Scope — Why It Matters and Best Practices
- 8 How Long Do Requirements Take?
- 9 How to Reuse Requirements Across Multiple Products
- 4. Requirements Traceability
- Overview
- 1 How is Traceability Achieved? A Practical Guide for Engineers
- 2 What is Requirements Traceability? Importance Explained
- 3 Tracing Your Way to Success: The Crucial Role of Traceability in Modern Product and Systems Development
- 4 Change Impact Analysis (CIA): A Short Guide for Effective Implementation
- 5 What is Meant by Version Control?
- 6 What is Requirements Traceability and Why Does It Matter for Product Teams?
- 7 Key Traceability Challenges and Tips for Ensuring Accountability and Efficiency
- 8 The Role of a Data Thread in Product and Software Development
- 9 Unraveling the Digital Thread: Enhancing Connectivity and Efficiency
- 10 Requirements Traceability Matrix (RTM): Definition and Purpose
- 11 How to Create and Use a Requirements Traceability Matrix (RTM)
- 12 Traceability Matrix 101: Why It’s Not the Ultimate Solution for Managing Requirements
- 13 Live Traceability vs. After-the-Fact Traceability
- 14 Overcoming Barriers to Live Requirements Traceability™
- 15 Requirements Traceability, What Are You Missing?
- 16 Four Best Practices for Requirements Traceability
- 17 Requirements Traceability: Links in the Chain
- 18 What Are the Benefits of End-to-End Traceability During Product Development?
- 19 FAQs About Requirements Traceability
- 5. Requirements Management Tools and Software
- Overview
- 1 Selecting the Right Requirements Management Tools and Software
- 2 Why Investing in Requirements Management Software Makes Business Sense During an Economic Downturn
- 3 Why Word and Excel Alone is Not Enough for Product, Software, and Systems Development
- 4 Application lifecycle management (ALM)
- 5 Is There Life After DOORS®?
- 6 Can You Track Requirements in Jira?
- 7 Checklist: Selecting a Requirements Management Tool
- 6. Requirements Validation and Verification
- 7. Meeting Regulatory Compliance and Industry Standards
- Overview
- 1 Understanding ISO Standards
- 2 Understanding ISO/IEC 27001: A Guide to Information Security Management
- 3 What is DevSecOps? A Guide to Building Secure Software
- 4 Compliance Management
- 5 What is FMEA? Failure Modes and Effects Analysis
- 6 TÜV SÜD: Ensuring Safety, Quality, and Sustainability Worldwide
- 8. Systems Engineering
- Overview
- 1 What is Systems Engineering?
- 2 How Do Engineers Collaborate? A Guide to Streamlined Teamwork and Innovation
- 3 The Systems Engineering Body of Knowledge (SEBoK)
- 4 What is MBSE? Model-Based Systems Engineering Explained
- 5 Digital Engineering Between Government and Contractors
- 6 Digital Engineering Tools: The Key to Driving Innovation and Efficiency in Complex Systems
- 9. Automotive Development
- 10. Medical Device & Life Sciences Development
- Overview
- 1 The Importance of Benefit-Risk Analysis in Medical Device Development
- 2 Software as a Medical Device: Revolutionizing Healthcare
- 3 What’s a Design History File, and How Are DHFs Used by Product Teams?
- 4 Navigating the Risks of Software of Unknown Pedigree (SOUP) in the Medical Device & Life Sciences Industry
- 5 What is ISO 13485? Your Comprehensive Guide to Compliant Medical Device Manufacturing
- 6 What You Need to Know: ANSI/AAMI SW96:2023 — Medical Device Security
- 7 ISO 13485 vs ISO 9001: Understanding the Differences and Synergies
- 8 Failure Modes, Effects, and Diagnostic Analysis (FMEDA) for Medical Devices: What You Need to Know
- 9 Embracing the Future of Healthcare: Exploring the Internet of Medical Things (IoMT)
- 11. Aerospace & Defense Development
- 12. Architecture, Engineering, and Construction (AEC industry) Development
- 13. Industrial Manufacturing & Machinery, Automation & Robotics, Consumer Electronics, and Energy
- 14. Semiconductor Development
- 15. AI in Product Development
- Glossary
Chapter 4: What is Requirements Traceability? Importance Explained
Chapters
- 1. Requirements Management
- Overview
- 1 What is Requirements Management?
- 2 Why do you need Requirements Management?
- 3 Four Stages of Requirements Management Processes
- 4 Adopting an Agile Approach to Requirements Management
- 5 Status Request Changes
- 6 Conquering the 5 Biggest Challenges of Requirements Management
- 7 Three Reasons You Need a Requirements Management Solution
- 8 Guide to Poor Requirements: Identify Causes, Repercussions, and How to Fix Them
- 2. Writing Requirements
- Overview
- 1 Functional requirements examples and templates
- 2 Identifying and Measuring Requirements Quality
- 3 How to write system requirement specification (SRS) documents
- 4 The Fundamentals of Business Requirements: Examples of Business Requirements and the Importance of Excellence
- 5 Adopting the EARS Notation to Improve Requirements Engineering
- 6 Jama Connect Advisor™
- 7 Frequently Asked Questions about the EARS Notation and Jama Connect Advisor™
- 8 How to Write an Effective Product Requirements Document (PRD)
- 9 Functional vs. Non-Functional Requirements
- 10 What Are Nonfunctional Requirements and How Do They Impact Product Development?
- 11 Characteristics of Effective Software Requirements and Software Requirements Specifications (SRS)
- 12 8 Do’s and Don’ts for Writing Requirements
- 3. Requirements Gathering and Management Processes
- Overview
- 1 Requirements Engineering
- 2 Requirements Analysis
- 3 A Guide to Requirements Elicitation for Product Teams
- 4 Requirements Gathering Techniques for Agile Product Teams
- 5 What is Requirements Gathering in Software Engineering?
- 6 Defining and Implementing a Requirements Baseline
- 7 Managing Project Scope — Why It Matters and Best Practices
- 8 How Long Do Requirements Take?
- 9 How to Reuse Requirements Across Multiple Products
- 4. Requirements Traceability
- Overview
- 1 How is Traceability Achieved? A Practical Guide for Engineers
- 2 What is Requirements Traceability? Importance Explained
- 3 Tracing Your Way to Success: The Crucial Role of Traceability in Modern Product and Systems Development
- 4 Change Impact Analysis (CIA): A Short Guide for Effective Implementation
- 5 What is Meant by Version Control?
- 6 What is Requirements Traceability and Why Does It Matter for Product Teams?
- 7 Key Traceability Challenges and Tips for Ensuring Accountability and Efficiency
- 8 The Role of a Data Thread in Product and Software Development
- 9 Unraveling the Digital Thread: Enhancing Connectivity and Efficiency
- 10 Requirements Traceability Matrix (RTM): Definition and Purpose
- 11 How to Create and Use a Requirements Traceability Matrix (RTM)
- 12 Traceability Matrix 101: Why It’s Not the Ultimate Solution for Managing Requirements
- 13 Live Traceability vs. After-the-Fact Traceability
- 14 Overcoming Barriers to Live Requirements Traceability™
- 15 Requirements Traceability, What Are You Missing?
- 16 Four Best Practices for Requirements Traceability
- 17 Requirements Traceability: Links in the Chain
- 18 What Are the Benefits of End-to-End Traceability During Product Development?
- 19 FAQs About Requirements Traceability
- 5. Requirements Management Tools and Software
- Overview
- 1 Selecting the Right Requirements Management Tools and Software
- 2 Why Investing in Requirements Management Software Makes Business Sense During an Economic Downturn
- 3 Why Word and Excel Alone is Not Enough for Product, Software, and Systems Development
- 4 Application lifecycle management (ALM)
- 5 Is There Life After DOORS®?
- 6 Can You Track Requirements in Jira?
- 7 Checklist: Selecting a Requirements Management Tool
- 6. Requirements Validation and Verification
- 7. Meeting Regulatory Compliance and Industry Standards
- Overview
- 1 Understanding ISO Standards
- 2 Understanding ISO/IEC 27001: A Guide to Information Security Management
- 3 What is DevSecOps? A Guide to Building Secure Software
- 4 Compliance Management
- 5 What is FMEA? Failure Modes and Effects Analysis
- 6 TÜV SÜD: Ensuring Safety, Quality, and Sustainability Worldwide
- 8. Systems Engineering
- Overview
- 1 What is Systems Engineering?
- 2 How Do Engineers Collaborate? A Guide to Streamlined Teamwork and Innovation
- 3 The Systems Engineering Body of Knowledge (SEBoK)
- 4 What is MBSE? Model-Based Systems Engineering Explained
- 5 Digital Engineering Between Government and Contractors
- 6 Digital Engineering Tools: The Key to Driving Innovation and Efficiency in Complex Systems
- 9. Automotive Development
- 10. Medical Device & Life Sciences Development
- Overview
- 1 The Importance of Benefit-Risk Analysis in Medical Device Development
- 2 Software as a Medical Device: Revolutionizing Healthcare
- 3 What’s a Design History File, and How Are DHFs Used by Product Teams?
- 4 Navigating the Risks of Software of Unknown Pedigree (SOUP) in the Medical Device & Life Sciences Industry
- 5 What is ISO 13485? Your Comprehensive Guide to Compliant Medical Device Manufacturing
- 6 What You Need to Know: ANSI/AAMI SW96:2023 — Medical Device Security
- 7 ISO 13485 vs ISO 9001: Understanding the Differences and Synergies
- 8 Failure Modes, Effects, and Diagnostic Analysis (FMEDA) for Medical Devices: What You Need to Know
- 9 Embracing the Future of Healthcare: Exploring the Internet of Medical Things (IoMT)
- 11. Aerospace & Defense Development
- 12. Architecture, Engineering, and Construction (AEC industry) Development
- 13. Industrial Manufacturing & Machinery, Automation & Robotics, Consumer Electronics, and Energy
- 14. Semiconductor Development
- 15. AI in Product Development
- Glossary
What is Requirements Traceability? Importance Explained
Requirements traceability is a core discipline in product and systems development that connects the dots between what you intend to build and what you actually deliver. As a fundamental part of the broader requirements management process detailed in our Chapter 4 Overview, it creates a living map of your project. This map links individual requirements to other development artifacts like tests, risks, and design specifications. For product teams, especially those in highly regulated industries such as automotive, aerospace, and medical devices, this practice is not just beneficial—it is essential. Without a clear line of sight from a product’s conception to its validation, teams risk costly rework, compliance issues, and project delays. Effective requirements traceability provides end-to-end visibility across the entire lifecycle, ensuring that every decision and action aligns with the initial goals and stakeholder needs.
What is meant by requirements traceability?

At its core, requirements traceability is the ability to follow the life of a requirement both forward and backward. It involves linking each requirement upstream to its origin—like a business need or stakeholder request—and downstream to its corresponding design elements, test cases, and validation activities. This creates an unbroken thread of information, providing end-to-end visibility across the development lifecycle. This comprehensive view ensures complete requirement coverage, confirming that every requirement is addressed and validated, leaving no gaps that could introduce risk or quality issues later on.
RELATED ARTICLE: Write Better Requirements with Jama Connect Advisor™
Ready to Find Out More?
Our team of experts is here to answer any questions and learn how we can help enable your continued success. Get started with a free 30-day trial, or book a demo!
Why is requirements traceability so important?
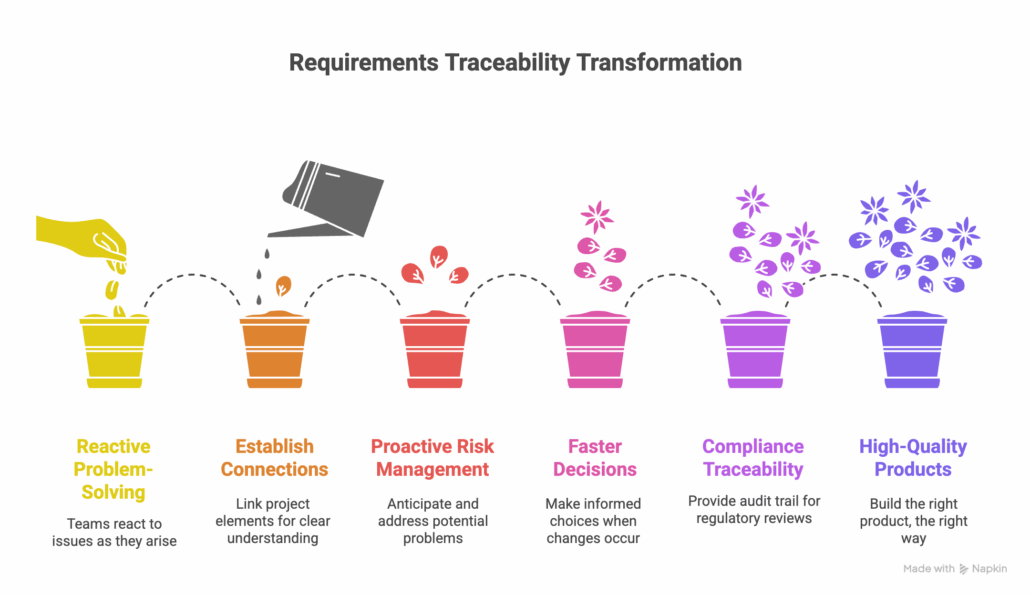
The importance of requirements traceability lies in its ability to bring clarity, control, and confidence to complex projects. It moves teams from reactive problem-solving to proactive risk management. By maintaining a clear connection between all project elements, teams can make faster, more informed decisions when changes occur. This enhances compliance traceability by providing a clear audit trail for regulatory reviews and simplifies the process of proving that all standards have been met. Ultimately, robust lifecycle management powered by traceability leads to better team collaboration and higher-quality products.
Key benefits include:
- Simplified Regulatory Compliance: Easily demonstrate that every standard and regulation has been met.
- Improved Impact Analysis: Quickly understand how a change to one requirement affects the entire system.
- Reduced Errors and Rework: Catch inconsistencies and gaps early in the development process.
- Enhanced Team Collaboration: Provide a single source of truth for all cross-functional teams.
Ultimately, strong requirements traceability is foundational to building the right product, the right way. The next step is understanding the difference between a manual approach and a dynamic one, which is covered in 4.2 Live Traceability vs. After-the-Fact.
RELATED ARTICLE: Requirements Traceability Benchmark
Experience the Power of Live Traceability™ with Jama Connect®
Requirements Traceability: is the tracking of requirements throughout the product development lifecycle.
Book a Demo
See Jama Connect in Action!
Our Jama Connect experts are ready to guide you through a personalized demo, answer your questions, and show you how Jama Connect can help you identify risks, improve cross-team collaboration, and drive faster time to market.