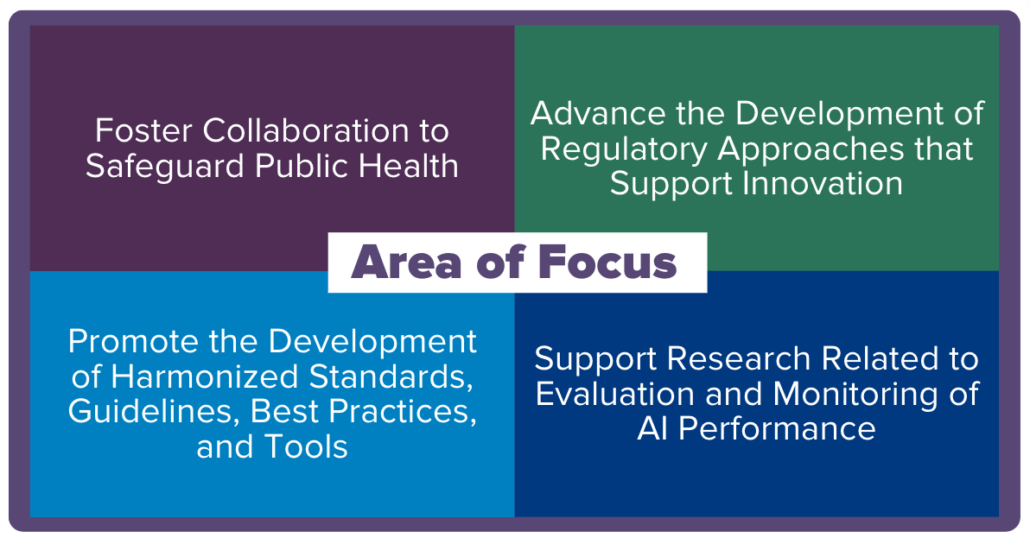
Jama Software is always looking for news that will benefit and inform our industry partners. As such, we’ve curated a series of customer and industry spotlight articles that we found insightful. In this blog post, we share an article, sourced from the U.S. Food & Drug Administration, titled “Ramping Up Security to Meet Operational Resilience Rules” – originally published on April 29, 2024.
FDA Takes Action Aimed at Helping to Ensure the Safety and Effectiveness of Laboratory Developed Tests
Today, the U.S. Food and Drug Administration took action aimed at helping to ensure the safety and effectiveness of laboratory developed tests, or LDTs, which are used in a growing number of health care decisions and about which concerns have been raised for many years.
LDTs are in vitro diagnostic products (IVDs) that the FDA has described as intended for clinical use and designed, manufactured and used within a single clinical laboratory that meets certain regulatory requirements. IVDs can play an important role in health care; they are used in the collection, preparation and examination of specimens taken from the human body, such as blood, saliva or tissue. They can be used to measure or detect substances or analytes, such as proteins, glucose, cholesterol or DNA, to provide information about a patient’s health, including to identify, monitor or determine treatment for diseases and conditions.
The FDA announced a final rule today amending the FDA’s regulations to make explicit that IVDs are devices under the Federal Food, Drug, and Cosmetic Act (FD&C Act) including when the manufacturer of the IVD is a laboratory. Along with this amendment, the FDA issued a policy to phase out, over the course of four years, its general enforcement discretion approach for LDTs. The agency also issued targeted enforcement discretion policies for certain categories of IVDs manufactured by laboratories.
“LDTs are being used more widely than ever before – for use in newborn screening, to help predict a person’s risk of cancer, or aid in diagnosing heart disease and Alzheimer’s. The agency cannot stand by while Americans continue to rely on results of these tests without assurance that they work,” said FDA Commissioner Robert M. Califf, M.D. “The final rule announced today aims to provide crucial oversight of these tests to help ensure that important health care decisions are made based on test results that patients and health care providers can trust.”
RELATED: Jama Connect® for Medical Device & Life Sciences Development Datasheet
Although historically the FDA has generally exercised enforcement discretion for most LDTs, meaning that the agency generally has not enforced applicable requirements with respect to most LDTs, the risks associated with most modern LDTs are much greater than the risks associated with LDTs used when the FDA’s enforcement discretion approach was adopted many decades ago. At that time, many LDTs were lower risk, small volume and used for specialized needs of a local patient population. Now, many LDTs are used more widely, for a larger and more diverse population, with large laboratories accepting specimens from across the country. LDTs also increasingly rely on high-tech instrumentation and software, are performed in large volumes and are used more frequently to help guide critical health care decisions.
Moreover, there is a growing body of evidence that demonstrates that some IVDs offered as LDTs raise public health concerns; for example, they do not provide accurate test results or do not perform as well as FDA-authorized tests, including from published studies in the scientific literature, the FDA’s own experience in reviewing IVDs offered as LDTs, news articles and class-action lawsuits.
The FDA is aware of numerous examples of potentially inaccurate, unsafe, ineffective or poor quality IVDs offered as LDTs that caused or may have caused patient harm, including tests used to select cancer treatment, aid in the diagnosis of COVID-19, aid in the management of patients with rare diseases and identify a patient’s risk of cancer.
Without greater oversight of the safety and effectiveness of LDTs, patients may be more likely to initiate unnecessary treatment, or delay or forego proper treatment based on inaccurate test results or tests promoted with false or misleading claims. This could result in harm, including worsening illness or death, as well as unnecessarily increase health care costs.
Increased compliance with device requirements under the FD&C Act (such as premarket review, quality system (QS) requirements, adverse event reporting, establishment registration and device listing, labeling requirements and investigational use requirements) will put patients and health care providers in a better position to have confidence in IVDs regardless of where they are manufactured.
With increased oversight, the FDA will also be able to help promote adequate representation in validation studies, as well as transparency regarding potential differential performance and unknown performance in certain patient populations, which may ultimately help advance health equity.
“Today’s action is a critical step toward helping to ensure the safety and effectiveness of LDTs, while also taking into account other public health considerations, including continued access to critical tests patients rely upon,” said Jeff Shuren, M.D., J.D., director of the FDA’s Center for Devices and Radiological Health. “Through targeted enforcement discretion policies for certain categories of tests manufactured by a laboratory, we expect patients and health care professionals will continue to have access to the tests they need while having greater confidence that the tests they rely on are accurate.”
The phaseout of the FDA’s general enforcement discretion approach for LDTs over a period of four years will protect the public health by helping to assure the safety and effectiveness of these tests, while avoiding undue disruption to patient care. Better assuring the safety and effectiveness of LDTs may also foster test innovation and facilitate the collective efforts of the scientific and medical communities to identify promising technologies, new therapies or areas worthy of future research.
Importantly, the FDA considered the large volume of comments received on the notice of proposed rulemaking, and in light of that input, has adjusted the phaseout policy in a manner that better serves the public health. After this phaseout, the FDA generally will expect IVDs made by either a non-laboratory or laboratory to meet the same requirements, though certain IVDs manufactured by laboratories may fall within one of the agency’s targeted enforcement discretion policies.
The FDA intends to exercise enforcement discretion with regard to premarket review and most quality system requirements for certain categories of IVDs, including but not limited to:
- Currently marketed IVDs offered as LDTs that were first marketed prior to the date of issuance of the final rule. This enforcement discretion policy is intended to address the risk that the perceived costs of compliance with such requirements could lead to the widespread loss of access to beneficial IVDs on which patients currently rely.
- LDTs manufactured and performed by a laboratory integrated within a health care system to meet an unmet need of patients receiving care within the same health care system when an FDA-authorized test is not available. This enforcement discretion policy is intended to help avoid patients being deprived of critically needed LDTs where certain risk mitigations exist that may help laboratories to identify any problems with their LDT and may help inform appropriate use and interpretation of such LDTs.
The FDA has also included additional enforcement discretion policies, such as for LDTs approved by the New York State’s Clinical Laboratory Evaluation Program (CLEP), as described in the preamble to the final rule, where that program’s review of analytical and clinical validity helps to mitigate the risk of harm from inaccurate and unreliable LDTs.
RELATED: Buyer’s Guide: Selecting a Requirements Management and Traceability Solution for Medical Device & Life Sciences
Draft Guidance Documents
The agency also issued two draft guidances today. One provides the agency’s thinking about an enforcement discretion policy for certain laboratories offering certain unauthorized IVDs for immediate response to an emergent situation, such as an outbreak of an infectious disease, in the absence of a declaration applicable to IVDs under section 564 of the FD&C Act. The other provides insight into the FDA’s thinking about the factors the agency intends to consider when developing a policy regarding enforcement discretion for certain IVDs during a public health emergency declared under section 564 of the FD&C Act.






![[Webinar Recap] Leveraging Jira & Jama Connect® for Enhanced Requirements Management [Webinar Recap] Leveraging Jira & Jama Connect® for Enhanced Requirements Management](https://www.jamasoftware.com/media/2024/04/Beyond-Jira-Word-Elevating-Requirements-Management-1.png)



![[Webinar Recap] Concurrent Engineering in Aerospace and Live Traceability™ [Webinar Recap] Concurrent Engineering in Aerospace and Live Traceability™](https://www.jamasoftware.com/media/2024/04/Concurrent-Engineering-in-Aerospace-and-Live-Traceability™-1.png)