In this blog, we recap our eBook, “Buyer’s Guide: Selecting a Requirements Management and Traceability Solution for Aerospace” – To download the entire thing, click HERE.
Buyer’s Guide: Selecting a Requirements Management and Traceability Solution for Aerospace
Use a Single Platform to Accelerate Innovation in Aerospace Systems Development
Consistently meeting product security, reliability and safety requirements proves the top challenge for aerospace systems development lifecycles. Contract complexity, streams of new regulations and policies, in addition to the challenges presented by increasingly networked systems, add to the already onerous development processes. This can lead to unmet technical performance parameters or delayed airworthiness certification reviews which for commercial aviation today takes years, to possibly lengthen out even more.
Effective management of these shifting complexities impacts your ability to meet compliance and ultimately, critical timelines. As a result, your development teams could find themselves:
- Mired in rework
- Making trade-offs without context
- Stuck in unproductive meetings
In this industry, aircraft and space systems development requires strict attention to safety and security requirements, as well as continuous innovation and fast paced development to remain competitive. Ineffective stakeholder collaboration and communication between suppliers, government customers, contractors, and vendors can lead to delivery delays and cost overruns.
Bottom Line: The accelerated development of safe, high-quality aerospace systems, coupled with a highly complex regulatory and contractual environment, create competing challenges, and make it difficult for teams to focus.
What if you didn’t have to compromise?
This Buyer’s Guide incorporates insights from Jama Software’s more than ten years of experience partnering with forward-thinking systems development teams. We’ve designed a platform to help aerospace systems development teams manage the systems engineering data and controls. This allows them to:
- Align to industry regulations quickly
- Simplify contract deliverables and certification preparations
- Accelerate time to market and mission
Use these insights to better understand the challenges you’re up against and thoughtfully consider potential solutions. Plus, learn how to get the buy-in you need to undertake the kind of transformation necessary to succeed with complex systems development.
Making the Case for Change
Jama Connect for aerospace systems development helps organizations to manage systems complexity and replace documents or legacy tools with a single digital platform. When requirements, architecture, V&V, and safety analyses are managed in a centralized location, contract deliverables and certification preparations become a straightforward process and the business impact and value of the platform becomes clear across the organization. That makes executive buy-in easier.
Corrective actions can cost anywhere from $1.6 million for a small change (Gulfstream Model G–1159A and G–1159B airplanes and all Model G–IV and GIV–X airplanes to remediate the ground spoiler actuator installation) to a large corrective action that has indirect costs of lost revenue and diminished market cap at over $20 billion (Boeing 737 MAX). Those costs are especially significant considering the price tag of system development – $75 million in FAA compliance alone—and an average timeline of three to seven years for type certification alone. For a space system, a failure can mean the entire loss of a system or spacecraft; typically there is only a single system created.
If your company is not considering the importance of transitioning to a more streamlined development process, time is not on your side. Failing to act quickly can leave your organization even further behind. But to see the value a positive impact a system can have, stakeholders in an organization have to appreciate the challenges first.
This is where you come in. You can help quantify the problem within your organization and provide data to help make the case for change.
Go through the exercises in the next section using data from your organization to identify your current situation and the size of the potential opportunity.
RELATED: CIMdata: Digital Thread in Aerospace and Defense
Tools to Assess Four Development Pain Points
Throughout the past decade of working with organizations developing complex aerospace systems, four common systems development pain points continuously arise for those who have yet to transform their process.
We’ll provide context around the problems and share equations with examples to help you uncover the savings from a modern systems development solution. Remember to adjust the variables according to your company’s metrics to get a more precise estimate, and rethink how your team functions.
Improving any one of these four aspects of your development process produces real savings. While the calculations on the following pages aren’t cumulative, they impact one another and can add up to significant value for your organization.
This is the potential of using a modern systems development platform. If realized, it can radically change your business and be the competitive edge you need in today’s market.
The Four Common Development Pain Points
- Unproductive Work Time
- Lengthy Time-to-Market
- Rework
- Defects
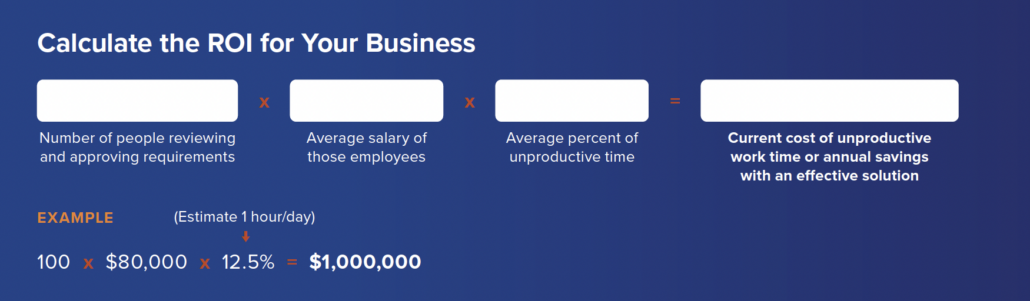
Unproductive Work time
Are your days spent in inefficient meetings, sifting through emails and document versions for historical information or waiting for reviews and approvals? You’re not alone. Many teams suffer the repercussions of archaic, siloed development. A modern process maximizes efficiency by tackling the root causes of momentum-killing delays and holdups.
Calculate how much unproductive work time is costing your business and imagine the possibilities of getting that time back. What could you do with one extra hour each day?
PRO TIP: We’ve seen long status meetings shrink or vanish when teams have the right solutions in place. Think about your team’s schedule and adjust the average time saved per person based on the time spent in meetings each week.
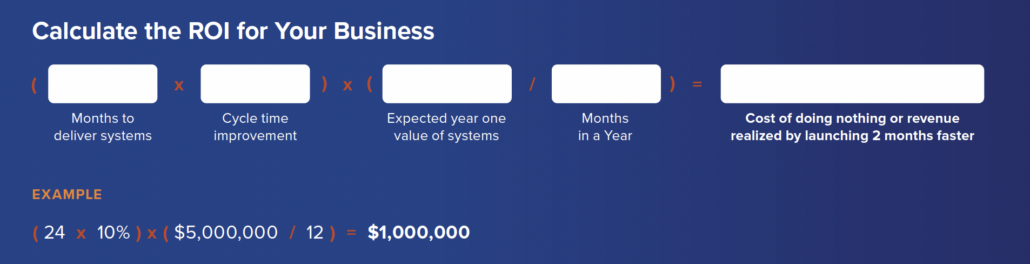
Lengthy Time-to-Mark
Time to market or meeting a mission deadline and quality are usually seen as compounding challenges. Understanding the impact of change, capturing decisions, communicating feedback and reusing
existing intellectual property — all aspects that can help speed time-to-market — can be improved with a modern systems development solution.
PRO TIP: Cost savings can certainly be great and have an impact on your bottom line, but don’t forget the qualitative implications. Consider what it would mean for your systems line and brand to be first-to-market with game-changing systems.
RELATED: Tracing Your Way to Success: The Crucial Role of Traceability in Modern Product and Systems Development
Rework
In our experience, approximately 30-50% of a given project is rework. Rework is any time spent on extra work — including mid-development changes, incorrect testing or fixing defects — and it costs your company big time. Requirements errors cause the majority of rework. Improving the ability to track requirements from definition through testing to catch changes and adjust scope can ensure
you’re building the right thing and massively reducing overall lifecycle costs.
Complete the equation below to get an understanding of the number of hours your team spends in rework and the value of that in work hours alone.
PRO TIP: If your organization is working on more than one system at a time, repeat this calculation for each and add up the savings for a holistic view.
Defects
It’s common for requirements to have a defect at some point between definition and delivery. The important thing is to have a system in place that can quickly and accurately identify defects and
track their impact up and downstream. This provides visibility into the problem as early as possible when it’s less detrimental to fix.
PRO TIP: This calculation factors in personnel hours, but you should also think about the cost of parts, delays, and missed opportunities. Plus, should defects go undetected due to sub-par requirements or testing, releasing lower-quality systems could have devastating consequences.
“A document-centric approach often requires a gatekeeper and really limits collaboration – that creates a bottleneck. With Jama Connect, all our development teams can work together from anywhere with a shared collaboration hub.” – David Cubbage, Director, LEO Satellite Engineering and Production, Telesat