Overcoming Barriers to Live Requirements Traceability™
The Essential Guide to Requirements Management and Traceability
Chapters
- 1. Requirements Management
- Overview
- 1 What is Requirements Management?
- 2 Why do you need Requirements Management?
- 3 Four Stages of Requirements Management Processes
- 4 Adopting an Agile Approach to Requirements Management
- 5 Status Request Changes
- 6 Conquering the 5 Biggest Challenges of Requirements Management
- 7 Three Reasons You Need a Requirements Management Solution
- 8 Guide to Poor Requirements: Identify Causes, Repercussions, and How to Fix Them
- 2. Writing Requirements
- Overview
- 1 Functional requirements examples and templates
- 2 Identifying and Measuring Requirements Quality
- 3 How to write system requirement specification (SRS) documents
- 4 The Fundamentals of Business Requirements: Examples of Business Requirements and the Importance of Excellence
- 5 Adopting the EARS Notation to Improve Requirements Engineering
- 6 Jama Connect Advisor™
- 7 Frequently Asked Questions about the EARS Notation and Jama Connect Advisor™
- 8 How to Write an Effective Product Requirements Document (PRD)
- 9 Functional vs. Non-Functional Requirements
- 10 What Are Nonfunctional Requirements and How Do They Impact Product Development?
- 11 Characteristics of Effective Software Requirements and Software Requirements Specifications (SRS)
- 12 8 Do’s and Don’ts for Writing Requirements
- 3. Requirements Gathering and Management Processes
- Overview
- 1 Requirements Engineering
- 2 Requirements Analysis
- 3 A Guide to Requirements Elicitation for Product Teams
- 4 Requirements Gathering Techniques for Agile Product Teams
- 5 What is Requirements Gathering?
- 6 Defining and Implementing a Requirements Baseline
- 7 Managing Project Scope — Why It Matters and Best Practices
- 8 How Long Do Requirements Take?
- 9 How to Reuse Requirements Across Multiple Products
- 4. Requirements Traceability
- Overview
- 1 What is Requirements Traceability? Importance Explained
- 2 How is Traceability Achieved? A Practical Guide for Engineers
- 3 Tracing Your Way to Success: The Crucial Role of Traceability in Modern Product and Systems Development
- 4 Change Impact Analysis (CIA): A Short Guide for Effective Implementation
- 5 What is Requirements Traceability and Why Does It Matter for Product Teams?
- 6 What is Meant by Version Control?
- 7 Key Traceability Challenges and Tips for Ensuring Accountability and Efficiency
- 8 Unraveling the Digital Thread: Enhancing Connectivity and Efficiency
- 9 The Role of a Data Thread in Product and Software Development
- 10 How to Create and Use a Requirements Traceability Matrix
- 11 Traceability Matrix 101: Why It’s Not the Ultimate Solution for Managing Requirements
- 12 Live Traceability vs. After-the-Fact Traceability
- 13 Overcoming Barriers to Live Requirements Traceability™
- 14 Requirements Traceability, What Are You Missing?
- 15 Four Best Practices for Requirements Traceability
- 16 Requirements Traceability: Links in the Chain
- 17 What Are the Benefits of End-to-End Traceability During Product Development?
- 5. Requirements Management Tools and Software
- Overview
- 1 Selecting the Right Requirements Management Tools and Software
- 2 Why Investing in Requirements Management Software Makes Business Sense During an Economic Downturn
- 3 Why Word and Excel Alone is Not Enough for Product, Software, and Systems Development
- 4 Application lifecycle management (ALM)
- 5 Is There Life After DOORS®?
- 6 Can You Track Requirements in Jira?
- 7 Checklist: Selecting a Requirements Management Tool
- 6. Requirements Validation and Verification
- 7. Meeting Regulatory Compliance and Industry Standards
- Overview
- 1 Understanding ISO Standards
- 2 Understanding ISO/IEC 27001: A Guide to Information Security Management
- 3 What is DevSecOps? A Guide to Building Secure Software
- 4 Compliance Management
- 5 What is FMEA? Failure Modes and Effects Analysis
- 6 TÜV SÜD: Ensuring Safety, Quality, and Sustainability Worldwide
- 8. Systems Engineering
- Overview
- 1 What is Systems Engineering?
- 2 How Do Engineers Collaborate? A Guide to Streamlined Teamwork and Innovation
- 3 The Systems Engineering Body of Knowledge (SEBoK)
- 4 What is MBSE? Model-Based Systems Engineering Explained
- 5 Digital Engineering Between Government and Contractors
- 6 Digital Engineering Tools: The Key to Driving Innovation and Efficiency in Complex Systems
- 9. Automotive Development
- 10. Medical Device & Life Sciences Development
- Overview
- 1 The Importance of Benefit-Risk Analysis in Medical Device Development
- 2 Software as a Medical Device: Revolutionizing Healthcare
- 3 What’s a Design History File, and How Are DHFs Used by Product Teams?
- 4 Navigating the Risks of Software of Unknown Pedigree (SOUP) in the Medical Device & Life Sciences Industry
- 5 What is ISO 13485? Your Comprehensive Guide to Compliant Medical Device Manufacturing
- 6 What You Need to Know: ANSI/AAMI SW96:2023 — Medical Device Security
- 7 ISO 13485 vs ISO 9001: Understanding the Differences and Synergies
- 8 Failure Modes, Effects, and Diagnostic Analysis (FMEDA) for Medical Devices: What You Need to Know
- 9 Embracing the Future of Healthcare: Exploring the Internet of Medical Things (IoMT)
- 11. Aerospace & Defense Development
- 12. Architecture, Engineering, and Construction (AEC industry) Development
- 13. Industrial Manufacturing & Machinery, Automation & Robotics, Consumer Electronics, and Energy
- 14. Semiconductor Development
- 15. AI in Product Development
- Glossary
Chapter 4: Overcoming Barriers to Live Requirements Traceability™
Chapters
- 1. Requirements Management
- Overview
- 1 What is Requirements Management?
- 2 Why do you need Requirements Management?
- 3 Four Stages of Requirements Management Processes
- 4 Adopting an Agile Approach to Requirements Management
- 5 Status Request Changes
- 6 Conquering the 5 Biggest Challenges of Requirements Management
- 7 Three Reasons You Need a Requirements Management Solution
- 8 Guide to Poor Requirements: Identify Causes, Repercussions, and How to Fix Them
- 2. Writing Requirements
- Overview
- 1 Functional requirements examples and templates
- 2 Identifying and Measuring Requirements Quality
- 3 How to write system requirement specification (SRS) documents
- 4 The Fundamentals of Business Requirements: Examples of Business Requirements and the Importance of Excellence
- 5 Adopting the EARS Notation to Improve Requirements Engineering
- 6 Jama Connect Advisor™
- 7 Frequently Asked Questions about the EARS Notation and Jama Connect Advisor™
- 8 How to Write an Effective Product Requirements Document (PRD)
- 9 Functional vs. Non-Functional Requirements
- 10 What Are Nonfunctional Requirements and How Do They Impact Product Development?
- 11 Characteristics of Effective Software Requirements and Software Requirements Specifications (SRS)
- 12 8 Do’s and Don’ts for Writing Requirements
- 3. Requirements Gathering and Management Processes
- Overview
- 1 Requirements Engineering
- 2 Requirements Analysis
- 3 A Guide to Requirements Elicitation for Product Teams
- 4 Requirements Gathering Techniques for Agile Product Teams
- 5 What is Requirements Gathering?
- 6 Defining and Implementing a Requirements Baseline
- 7 Managing Project Scope — Why It Matters and Best Practices
- 8 How Long Do Requirements Take?
- 9 How to Reuse Requirements Across Multiple Products
- 4. Requirements Traceability
- Overview
- 1 What is Requirements Traceability? Importance Explained
- 2 How is Traceability Achieved? A Practical Guide for Engineers
- 3 Tracing Your Way to Success: The Crucial Role of Traceability in Modern Product and Systems Development
- 4 Change Impact Analysis (CIA): A Short Guide for Effective Implementation
- 5 What is Requirements Traceability and Why Does It Matter for Product Teams?
- 6 What is Meant by Version Control?
- 7 Key Traceability Challenges and Tips for Ensuring Accountability and Efficiency
- 8 Unraveling the Digital Thread: Enhancing Connectivity and Efficiency
- 9 The Role of a Data Thread in Product and Software Development
- 10 How to Create and Use a Requirements Traceability Matrix
- 11 Traceability Matrix 101: Why It’s Not the Ultimate Solution for Managing Requirements
- 12 Live Traceability vs. After-the-Fact Traceability
- 13 Overcoming Barriers to Live Requirements Traceability™
- 14 Requirements Traceability, What Are You Missing?
- 15 Four Best Practices for Requirements Traceability
- 16 Requirements Traceability: Links in the Chain
- 17 What Are the Benefits of End-to-End Traceability During Product Development?
- 5. Requirements Management Tools and Software
- Overview
- 1 Selecting the Right Requirements Management Tools and Software
- 2 Why Investing in Requirements Management Software Makes Business Sense During an Economic Downturn
- 3 Why Word and Excel Alone is Not Enough for Product, Software, and Systems Development
- 4 Application lifecycle management (ALM)
- 5 Is There Life After DOORS®?
- 6 Can You Track Requirements in Jira?
- 7 Checklist: Selecting a Requirements Management Tool
- 6. Requirements Validation and Verification
- 7. Meeting Regulatory Compliance and Industry Standards
- Overview
- 1 Understanding ISO Standards
- 2 Understanding ISO/IEC 27001: A Guide to Information Security Management
- 3 What is DevSecOps? A Guide to Building Secure Software
- 4 Compliance Management
- 5 What is FMEA? Failure Modes and Effects Analysis
- 6 TÜV SÜD: Ensuring Safety, Quality, and Sustainability Worldwide
- 8. Systems Engineering
- Overview
- 1 What is Systems Engineering?
- 2 How Do Engineers Collaborate? A Guide to Streamlined Teamwork and Innovation
- 3 The Systems Engineering Body of Knowledge (SEBoK)
- 4 What is MBSE? Model-Based Systems Engineering Explained
- 5 Digital Engineering Between Government and Contractors
- 6 Digital Engineering Tools: The Key to Driving Innovation and Efficiency in Complex Systems
- 9. Automotive Development
- 10. Medical Device & Life Sciences Development
- Overview
- 1 The Importance of Benefit-Risk Analysis in Medical Device Development
- 2 Software as a Medical Device: Revolutionizing Healthcare
- 3 What’s a Design History File, and How Are DHFs Used by Product Teams?
- 4 Navigating the Risks of Software of Unknown Pedigree (SOUP) in the Medical Device & Life Sciences Industry
- 5 What is ISO 13485? Your Comprehensive Guide to Compliant Medical Device Manufacturing
- 6 What You Need to Know: ANSI/AAMI SW96:2023 — Medical Device Security
- 7 ISO 13485 vs ISO 9001: Understanding the Differences and Synergies
- 8 Failure Modes, Effects, and Diagnostic Analysis (FMEDA) for Medical Devices: What You Need to Know
- 9 Embracing the Future of Healthcare: Exploring the Internet of Medical Things (IoMT)
- 11. Aerospace & Defense Development
- 12. Architecture, Engineering, and Construction (AEC industry) Development
- 13. Industrial Manufacturing & Machinery, Automation & Robotics, Consumer Electronics, and Energy
- 14. Semiconductor Development
- 15. AI in Product Development
- Glossary
Overcoming Barriers to Live Requirements Traceability™
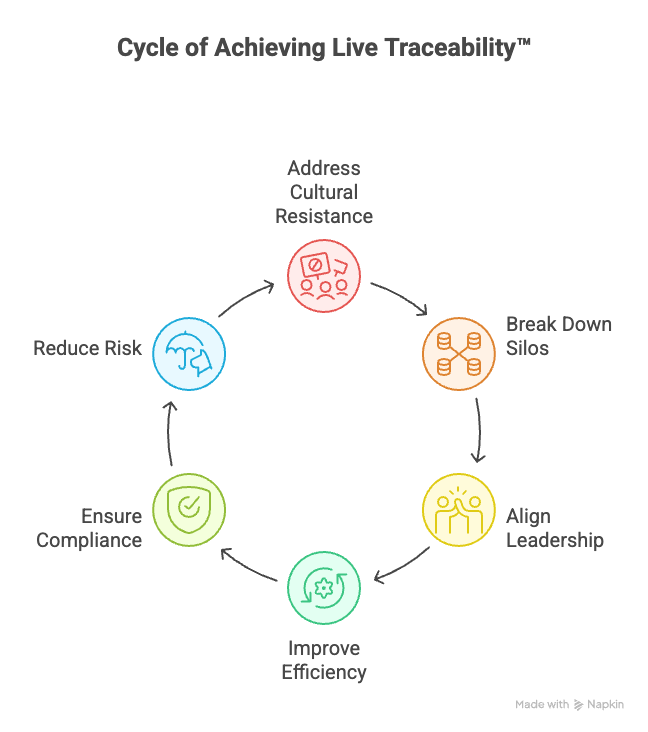
Achieving Live Requirements Traceability™ is essential for reducing risk, ensuring compliance, and improving product development efficiency. However, the biggest obstacles to adoption are often organizational rather than technical. Overcoming these barriers requires addressing cultural resistance, siloed teams, and leadership alignment to drive change.
Common Challenges in Adopting Live Traceability™
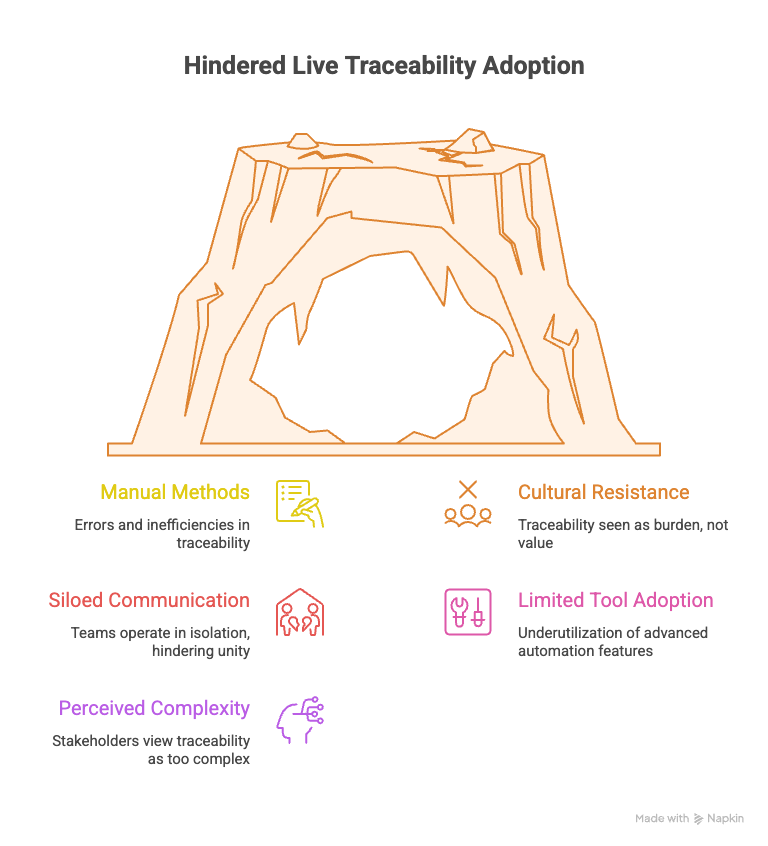
Organizations face several hurdles when implementing Live Traceability, many of which stem from entrenched processes and resistance to change. Key challenges include:
- Reliance on manual methods: Teams often depend on spreadsheets or after-the-fact documentation, leading to errors and inefficiencies.
- Cultural resistance to change: Employees may view traceability as an added burden rather than a value-driven process.
- Siloed communication: Engineering, compliance, and product teams frequently operate in isolation, making it difficult to establish a unified source of truth.
- Limited tool adoption: While organizations may have requirements tools, they often fail to leverage advanced features that enable automation.
- Perceived complexity: Stakeholders may see Live Traceability as too resource-intensive or complex to implement.
These challenges persist because they are deeply rooted in organizational habits and a lack of clear understanding of the benefits of Live Traceability. Addressing these issues is critical to achieving continuous compliance and reducing development risks.
RELATED ARTICLE: Live Traceability™ vs. After-the-Fact Traceability Explained
Ready to Find Out More?
Our team of experts is here to answer any questions and learn how we can help enable your continued success. Get started with a free 30-day trial, or book a demo!
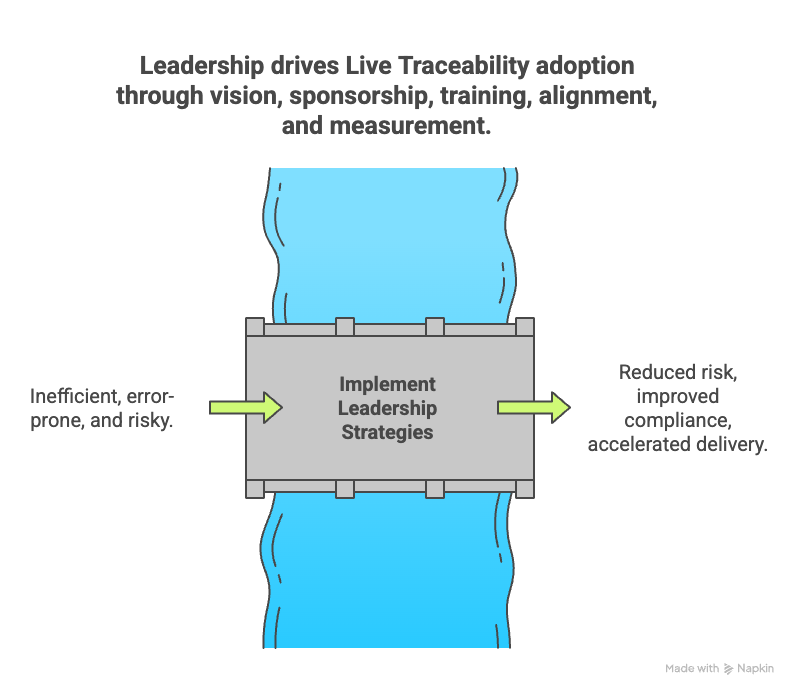
How leadership can drive automation and adoption
Leadership plays a pivotal role in overcoming organizational barriers and enabling Live Traceability. By setting a clear vision and fostering collaboration, leaders can drive meaningful change. Here’s how:
- Set a clear vision: Communicate how Live Traceability supports compliance, reduces risk, and accelerates delivery timelines.
- Secure executive sponsorship: Demonstrate ROI and business value to gain budget approval and long-term commitment.
- Provide training and enablement: Equip teams with the knowledge and tools needed to adopt automation effectively.
- Promote cross-functional alignment: Encourage collaboration between engineering, QA, compliance, and product teams to break down silos.
- Measure and celebrate progress: Track adoption metrics, highlight early wins, and share success stories to build momentum.
By taking these steps, leadership can create an environment where Live Traceability is not only achievable but also embraced across the organization.
RELATED ARTICLE: Requirements Traceability – How to Go Live
Check Out Our Webinar: Best Practices for Live Traceability™
Requirements Traceability: is the tracking of requirements throughout the product development lifecycle.
Book a Demo
See Jama Connect in Action!
Our Jama Connect experts are ready to guide you through a personalized demo, answer your questions, and show you how Jama Connect can help you identify risks, improve cross-team collaboration, and drive faster time to market.