Four Stages of Requirements Management Processes
The Essential Guide to Requirements Management and Traceability
Chapters
- 1. Requirements Management
- Overview
- 1 What is Requirements Management?
- 2 Why do you need Requirements Management?
- 3 Four Stages of Requirements Management Processes
- 4 Adopting an Agile Approach to Requirements Management
- 5 Status Request Changes
- 6 Conquering the 5 Biggest Challenges of Requirements Management
- 7 Three Reasons You Need a Requirements Management Solution
- 8 Guide to Poor Requirements: Identify Causes, Repercussions, and How to Fix Them
- 2. Writing Requirements
- Overview
- 1 Functional requirements examples and templates
- 2 Identifying and Measuring Requirements Quality
- 3 How to write system requirement specification (SRS) documents
- 4 The Fundamentals of Business Requirements: Examples of Business Requirements and the Importance of Excellence
- 5 Adopting the EARS Notation to Improve Requirements Engineering
- 6 Jama Connect Advisor™
- 7 Frequently Asked Questions about the EARS Notation and Jama Connect Advisor™
- 8 How to Write an Effective Product Requirements Document (PRD)
- 9 Functional vs. Non-Functional Requirements
- 10 What Are Nonfunctional Requirements and How Do They Impact Product Development?
- 11 Characteristics of Effective Software Requirements and Software Requirements Specifications (SRS)
- 12 8 Do’s and Don’ts for Writing Requirements
- 3. Requirements Gathering and Management Processes
- Overview
- 1 Requirements Engineering
- 2 Requirements Analysis
- 3 A Guide to Requirements Elicitation for Product Teams
- 4 Requirements Gathering Techniques for Agile Product Teams
- 5 What is Requirements Gathering in Software Engineering?
- 6 Defining and Implementing a Requirements Baseline
- 7 Managing Project Scope — Why It Matters and Best Practices
- 8 How Long Do Requirements Take?
- 9 How to Reuse Requirements Across Multiple Products
- 4. Requirements Traceability
- Overview
- 1 How is Traceability Achieved? A Practical Guide for Engineers
- 2 What is Requirements Traceability? Importance Explained
- 3 Tracing Your Way to Success: The Crucial Role of Traceability in Modern Product and Systems Development
- 4 Change Impact Analysis (CIA): A Short Guide for Effective Implementation
- 5 What is Meant by Version Control?
- 6 What is Requirements Traceability and Why Does It Matter for Product Teams?
- 7 Key Traceability Challenges and Tips for Ensuring Accountability and Efficiency
- 8 The Role of a Data Thread in Product and Software Development
- 9 Unraveling the Digital Thread: Enhancing Connectivity and Efficiency
- 10 Requirements Traceability Matrix (RTM): Definition and Purpose
- 11 How to Create and Use a Requirements Traceability Matrix (RTM)
- 12 Traceability Matrix 101: Why It’s Not the Ultimate Solution for Managing Requirements
- 13 Live Traceability vs. After-the-Fact Traceability
- 14 Overcoming Barriers to Live Requirements Traceability™
- 15 Requirements Traceability, What Are You Missing?
- 16 Four Best Practices for Requirements Traceability
- 17 Requirements Traceability: Links in the Chain
- 18 What Are the Benefits of End-to-End Traceability During Product Development?
- 19 FAQs About Requirements Traceability
- 5. Requirements Management Tools and Software
- Overview
- 1 Selecting the Right Requirements Management Tools and Software
- 2 Why Investing in Requirements Management Software Makes Business Sense During an Economic Downturn
- 3 Why Word and Excel Alone is Not Enough for Product, Software, and Systems Development
- 4 Application lifecycle management (ALM)
- 5 Is There Life After DOORS®?
- 6 Can You Track Requirements in Jira?
- 7 Checklist: Selecting a Requirements Management Tool
- 6. Requirements Validation and Verification
- 7. Meeting Regulatory Compliance and Industry Standards
- Overview
- 1 Understanding ISO Standards
- 2 Understanding ISO/IEC 27001: A Guide to Information Security Management
- 3 What is DevSecOps? A Guide to Building Secure Software
- 4 Compliance Management
- 5 What is FMEA? Failure Modes and Effects Analysis
- 6 TÜV SÜD: Ensuring Safety, Quality, and Sustainability Worldwide
- 8. Systems Engineering
- Overview
- 1 What is Systems Engineering?
- 2 How Do Engineers Collaborate? A Guide to Streamlined Teamwork and Innovation
- 3 The Systems Engineering Body of Knowledge (SEBoK)
- 4 What is MBSE? Model-Based Systems Engineering Explained
- 5 Digital Engineering Between Government and Contractors
- 6 Digital Engineering Tools: The Key to Driving Innovation and Efficiency in Complex Systems
- 9. Automotive Development
- 10. Medical Device & Life Sciences Development
- Overview
- 1 The Importance of Benefit-Risk Analysis in Medical Device Development
- 2 Software as a Medical Device: Revolutionizing Healthcare
- 3 What’s a Design History File, and How Are DHFs Used by Product Teams?
- 4 Navigating the Risks of Software of Unknown Pedigree (SOUP) in the Medical Device & Life Sciences Industry
- 5 What is ISO 13485? Your Comprehensive Guide to Compliant Medical Device Manufacturing
- 6 What You Need to Know: ANSI/AAMI SW96:2023 — Medical Device Security
- 7 ISO 13485 vs ISO 9001: Understanding the Differences and Synergies
- 8 Failure Modes, Effects, and Diagnostic Analysis (FMEDA) for Medical Devices: What You Need to Know
- 9 Embracing the Future of Healthcare: Exploring the Internet of Medical Things (IoMT)
- 11. Aerospace & Defense Development
- 12. Architecture, Engineering, and Construction (AEC industry) Development
- 13. Industrial Manufacturing & Machinery, Automation & Robotics, Consumer Electronics, and Energy
- 14. Semiconductor Development
- 15. AI in Product Development
- Glossary
Chapter 1: Four Stages of Requirements Management Processes
Chapters
- 1. Requirements Management
- Overview
- 1 What is Requirements Management?
- 2 Why do you need Requirements Management?
- 3 Four Stages of Requirements Management Processes
- 4 Adopting an Agile Approach to Requirements Management
- 5 Status Request Changes
- 6 Conquering the 5 Biggest Challenges of Requirements Management
- 7 Three Reasons You Need a Requirements Management Solution
- 8 Guide to Poor Requirements: Identify Causes, Repercussions, and How to Fix Them
- 2. Writing Requirements
- Overview
- 1 Functional requirements examples and templates
- 2 Identifying and Measuring Requirements Quality
- 3 How to write system requirement specification (SRS) documents
- 4 The Fundamentals of Business Requirements: Examples of Business Requirements and the Importance of Excellence
- 5 Adopting the EARS Notation to Improve Requirements Engineering
- 6 Jama Connect Advisor™
- 7 Frequently Asked Questions about the EARS Notation and Jama Connect Advisor™
- 8 How to Write an Effective Product Requirements Document (PRD)
- 9 Functional vs. Non-Functional Requirements
- 10 What Are Nonfunctional Requirements and How Do They Impact Product Development?
- 11 Characteristics of Effective Software Requirements and Software Requirements Specifications (SRS)
- 12 8 Do’s and Don’ts for Writing Requirements
- 3. Requirements Gathering and Management Processes
- Overview
- 1 Requirements Engineering
- 2 Requirements Analysis
- 3 A Guide to Requirements Elicitation for Product Teams
- 4 Requirements Gathering Techniques for Agile Product Teams
- 5 What is Requirements Gathering in Software Engineering?
- 6 Defining and Implementing a Requirements Baseline
- 7 Managing Project Scope — Why It Matters and Best Practices
- 8 How Long Do Requirements Take?
- 9 How to Reuse Requirements Across Multiple Products
- 4. Requirements Traceability
- Overview
- 1 How is Traceability Achieved? A Practical Guide for Engineers
- 2 What is Requirements Traceability? Importance Explained
- 3 Tracing Your Way to Success: The Crucial Role of Traceability in Modern Product and Systems Development
- 4 Change Impact Analysis (CIA): A Short Guide for Effective Implementation
- 5 What is Meant by Version Control?
- 6 What is Requirements Traceability and Why Does It Matter for Product Teams?
- 7 Key Traceability Challenges and Tips for Ensuring Accountability and Efficiency
- 8 The Role of a Data Thread in Product and Software Development
- 9 Unraveling the Digital Thread: Enhancing Connectivity and Efficiency
- 10 Requirements Traceability Matrix (RTM): Definition and Purpose
- 11 How to Create and Use a Requirements Traceability Matrix (RTM)
- 12 Traceability Matrix 101: Why It’s Not the Ultimate Solution for Managing Requirements
- 13 Live Traceability vs. After-the-Fact Traceability
- 14 Overcoming Barriers to Live Requirements Traceability™
- 15 Requirements Traceability, What Are You Missing?
- 16 Four Best Practices for Requirements Traceability
- 17 Requirements Traceability: Links in the Chain
- 18 What Are the Benefits of End-to-End Traceability During Product Development?
- 19 FAQs About Requirements Traceability
- 5. Requirements Management Tools and Software
- Overview
- 1 Selecting the Right Requirements Management Tools and Software
- 2 Why Investing in Requirements Management Software Makes Business Sense During an Economic Downturn
- 3 Why Word and Excel Alone is Not Enough for Product, Software, and Systems Development
- 4 Application lifecycle management (ALM)
- 5 Is There Life After DOORS®?
- 6 Can You Track Requirements in Jira?
- 7 Checklist: Selecting a Requirements Management Tool
- 6. Requirements Validation and Verification
- 7. Meeting Regulatory Compliance and Industry Standards
- Overview
- 1 Understanding ISO Standards
- 2 Understanding ISO/IEC 27001: A Guide to Information Security Management
- 3 What is DevSecOps? A Guide to Building Secure Software
- 4 Compliance Management
- 5 What is FMEA? Failure Modes and Effects Analysis
- 6 TÜV SÜD: Ensuring Safety, Quality, and Sustainability Worldwide
- 8. Systems Engineering
- Overview
- 1 What is Systems Engineering?
- 2 How Do Engineers Collaborate? A Guide to Streamlined Teamwork and Innovation
- 3 The Systems Engineering Body of Knowledge (SEBoK)
- 4 What is MBSE? Model-Based Systems Engineering Explained
- 5 Digital Engineering Between Government and Contractors
- 6 Digital Engineering Tools: The Key to Driving Innovation and Efficiency in Complex Systems
- 9. Automotive Development
- 10. Medical Device & Life Sciences Development
- Overview
- 1 The Importance of Benefit-Risk Analysis in Medical Device Development
- 2 Software as a Medical Device: Revolutionizing Healthcare
- 3 What’s a Design History File, and How Are DHFs Used by Product Teams?
- 4 Navigating the Risks of Software of Unknown Pedigree (SOUP) in the Medical Device & Life Sciences Industry
- 5 What is ISO 13485? Your Comprehensive Guide to Compliant Medical Device Manufacturing
- 6 What You Need to Know: ANSI/AAMI SW96:2023 — Medical Device Security
- 7 ISO 13485 vs ISO 9001: Understanding the Differences and Synergies
- 8 Failure Modes, Effects, and Diagnostic Analysis (FMEDA) for Medical Devices: What You Need to Know
- 9 Embracing the Future of Healthcare: Exploring the Internet of Medical Things (IoMT)
- 11. Aerospace & Defense Development
- 12. Architecture, Engineering, and Construction (AEC industry) Development
- 13. Industrial Manufacturing & Machinery, Automation & Robotics, Consumer Electronics, and Energy
- 14. Semiconductor Development
- 15. AI in Product Development
- Glossary
Four Stages of Requirements Management Processes
A solid requirements management process is the foundation of any successful product development. Without it, teams risk miscommunication, scope creep, and costly rework that can derail timelines and budgets. A structured approach ensures that evolving requirements are managed consistently, leading to a higher-quality product that meets stakeholder expectations.
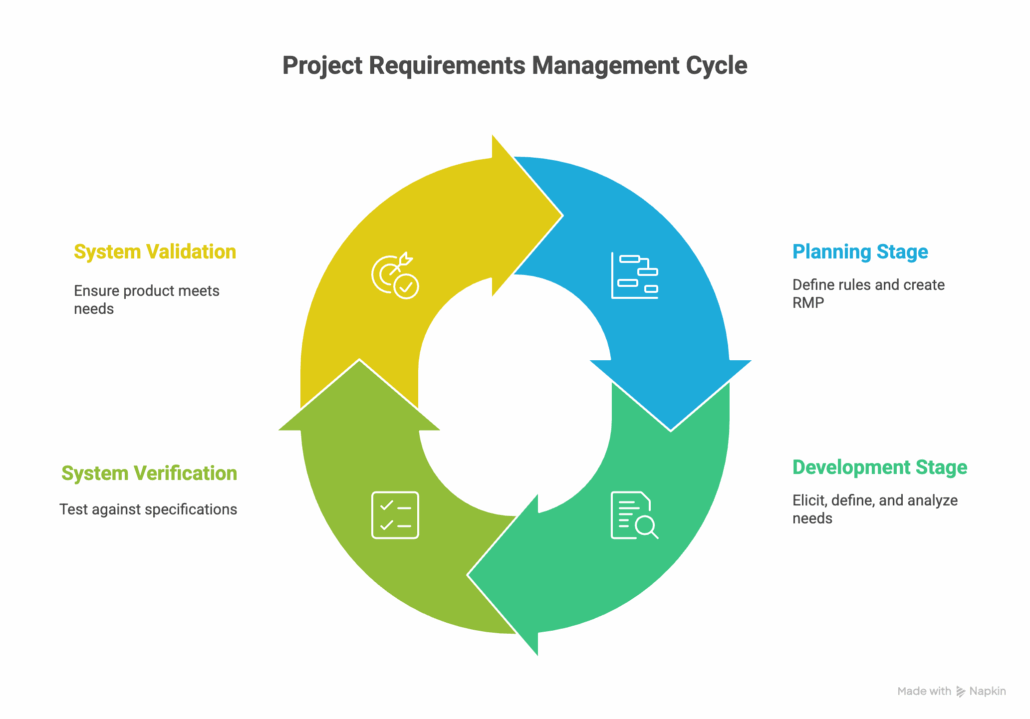
The process can be broken into four key stages: planning, development, verification, and validation. Change management is not a separate stage but a continuous activity throughout the lifecycle, ensuring all requirements remain traceable and adaptable.
TL;DR: Mastering these four stages helps you build the right product, the right way.
Question: How mature is your current requirements management process?
RELATED ARTICLE: Requirements Management Tools and Software
Stage 1: The Planning Stage
The planning stage sets the foundation for the entire project. This is where you define the rules of engagement for how your team will handle requirements from start to finish. The development methodology your team uses—be it Waterfall, Agile, or a hybrid approach—will heavily influence how requirements flow through the subsequent stages.
A key deliverable of this stage is the Requirements Management Plan (RMP). This document is the roadmap for your project and outlines:
- Stakeholder roles and responsibilities.
- The types of requirements artifacts to be created (e.g., use cases, design documents).
- How traceability will be established and maintained.
- The process for creating and managing baselines.
- The strategy for managing changes to requirements.
Key takeaway: Gaining stakeholder buy-in on the RMP is crucial. It aligns expectations and provides a clear path forward for everyone involved. Another critical part of planning is defining a requirements baseline—a snapshot of agreed-upon requirements that marks the start of formal change control.
Stage 2: The Development Stage
The development stage is where requirements are brought to life. This phase focuses on eliciting, defining, and analyzing the needs of all stakeholders to provide the development team with a clear and unambiguous set of instructions.
Key activities in this stage include:
- Eliciting Needs and Requirements: Work directly with customers, users, and internal stakeholders to gather their expectations for the product using techniques like interviews, workshops, and surveys.
- Defining Needs and Requirements: Translate gathered needs into clear, concise, and testable requirements. A high-quality requirement must be necessary, unambiguous, feasible, and verifiable.
- Requirements Analysis: Analyze, negotiate, and resolve any conflicting or unclear requirements to ensure the final set accurately reflects the project’s goals.
The most important part of this stage is establishing bi-directional traceability. This allows teams to see how a change to one requirement might impact others, as well as associated tests and design elements. This is especially critical in regulated industries where proving compliance is mandatory.
Stage 3: The System Verification Stage
System verification answers the question: “Did we build the product right?” This stage involves a series of tests and evaluations to confirm that the finished product meets the technical specifications and requirements defined in the baseline. Verification always comes before validation.
To ensure success, planning for verification should begin as the requirements are being written. For each product requirement, you should define:
- Success Criteria: What is the measurable outcome that proves the requirement has been met?
- Method: How will you verify it? Common methods include tests, demonstrations, inspections, or analyses.
- Strategy: What is the approach, including the environment and system configuration needed for testing?
Defining these attributes early sets clear expectations for the test and quality assurance teams and helps reduce the risk of discovering major issues late in the development cycle.
Stage 4: The System Validation Stage
While verification checks against specifications, system validation answers a more fundamental question: “Did we build the right product?” The goal here is to determine if the product fulfills its intended purpose and meets the original stakeholder needs in a real-world operational environment.
Key takeaway: Successful validation leads to user acceptance and, in regulated industries, official approval for use. Similar to verification, planning for validation should begin as soon as the initial stakeholder needs are defined, ensuring the end product not only works as designed but also solves the problem it was created to address.
RELATED ARTICLE: Requirements Traceability Benchmark
Ready to Find Out More?
Our team of experts is here to answer any questions and learn how we can help enable your continued success. Get started with a free 30-day trial, or book a demo!
Overcoming Common Process Challenges
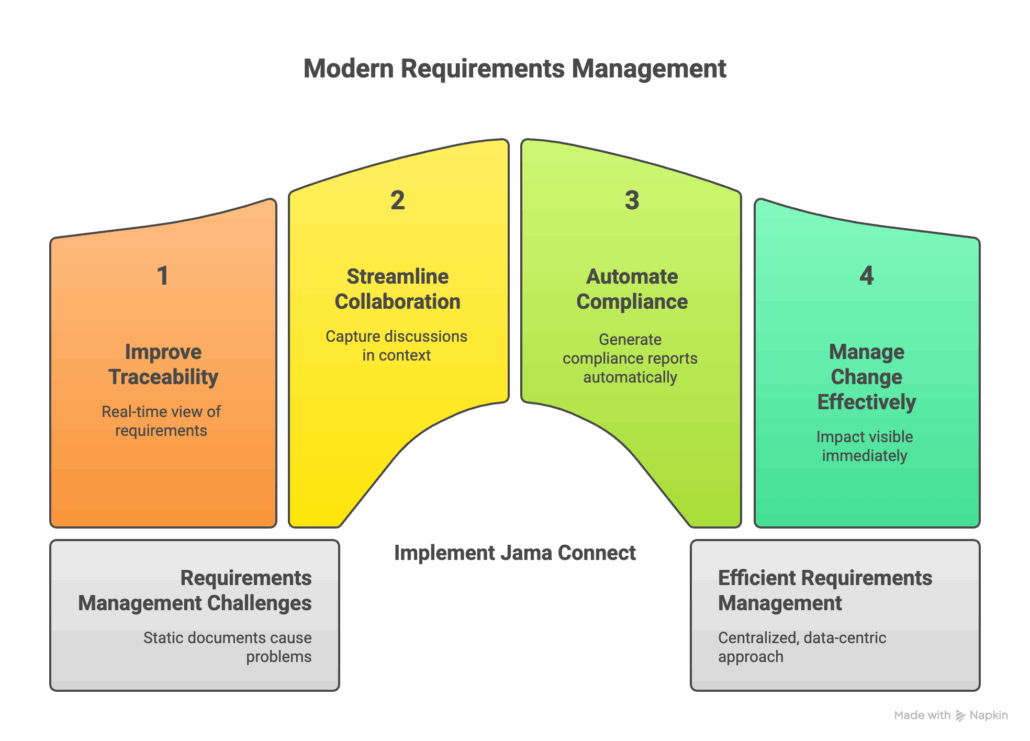
Every stage of the requirements management process presents potential challenges. Teams often struggle with last-minute feedback, decision rehashing, and mismatched stakeholder expectations. These problems are often magnified when teams rely on static documents like spreadsheets and Word files to manage requirements. Version control becomes a nightmare, and maintaining traceability across thousands of requirements is nearly impossible.
This is where modern requirements management tools make a significant difference. Platforms like Jama Connect® provide a centralized, data-centric approach to managing the entire process. By moving away from static documents, teams can:
- Improve Traceability: Live Traceability™ offers a real-time, bi-directional view of all requirements, tests, and related artifacts, making impact analysis simple and instant.
- Streamline Collaboration: Teams can collaborate in real-time, capturing discussions and decisions in context, which eliminates the need to hunt through emails and meeting notes.
- Automate Compliance: For teams in regulated industries, Jama Connect can simplify audit preparation by generating comprehensive compliance reports and maintaining a detailed digital audit trail automatically.
- Manage Change Effectively: When a requirement changes, the impact on upstream and downstream items is immediately visible, allowing teams to make informed decisions quickly.
FAQs
Q1: What is the most important stage in the requirements management process?
While all four stages are critical for success, the Planning Stage is arguably the most foundational. A well-thought-out plan, including a comprehensive RMP and a clear baseline, prevents many of the common issues that derail projects later on.
Q2: What is the difference between system verification and system validation?
Verification asks, “Did we build the product right?” It checks if the product meets its technical specifications. Validation asks, “Did we build the right product?” It confirms if the product meets the actual needs of the stakeholders in its intended environment.
Q3: How can a tool improve our requirements management process?
A dedicated tool like Jama Connect replaces error-prone documents with a centralized platform. This improves collaboration, provides live traceability to manage complexity, automates compliance documentation, and gives teams the visibility needed to manage change effectively.
Take Control of Your Requirements Management Process
A well-defined requirements management process is not just about documentation; it is about communication, collaboration, and control. By implementing these four stages and leveraging the right tools, you can ensure your team delivers high-quality products that meet user needs—on time and within budget.
See how Jama Connect can help you master your requirements management process.
[Request a Demo Today]
In This Webinar, Learn How AI Is Transforming Submission Readiness
Book a Demo
See Jama Connect in Action!
Our Jama Connect experts are ready to guide you through a personalized demo, answer your questions, and show you how Jama Connect can help you identify risks, improve cross-team collaboration, and drive faster time to market.