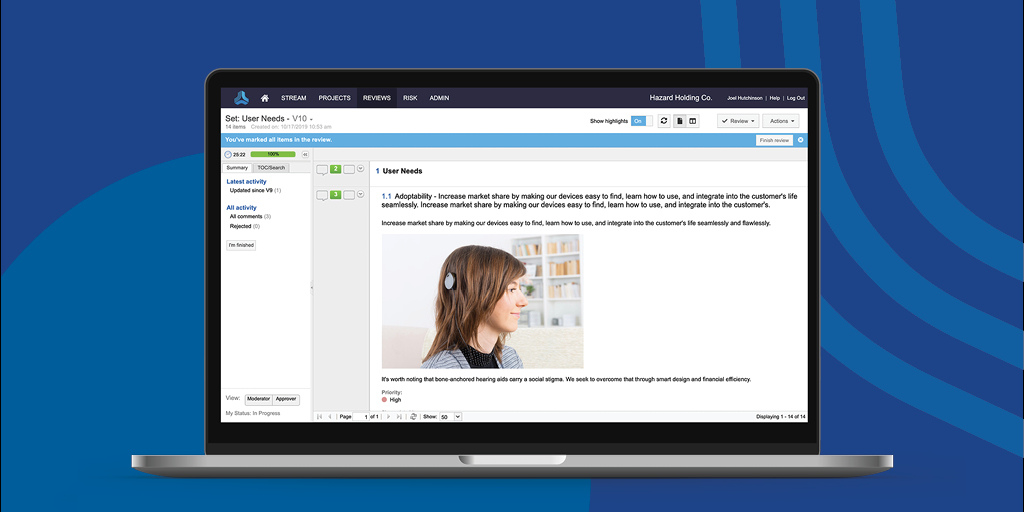
Reviews play a key role in successful product and systems development, helping to ensure a new project meets stakeholder, market, and compliance requirements.
By taking an iterative and collaborative approach to reviewing requirements and tests in real-time, Jama Connect™ Review Center improves stakeholder alignment, reduces lengthy review cycles, and eases the path to compliance.
To that end, we are excited to announce today that we have made significant improvements to Review Center, an already beloved feature of the Jama Connect platform, which are available now to our customers.
Within Jama Connect Review Center, users can now streamline reviews and ease the path to regulatory compliance thanks to:
- A new Review Center Wizard that streamlines review set up for particular objectives
- Simplified application, management, and accessibility of electronic signatures for reviews
- Easier configuration of review settings, such as ones used to apply electronic signatures per FDA 21 CFR Part 11 to ensure they can be properly referenced and signed off on across different teams.
Additionally, customers can now add electronic signature roles, prevent reviews with e-signatures from being deleted, view review activity history for audits, and better set expectations for participants based on their role.
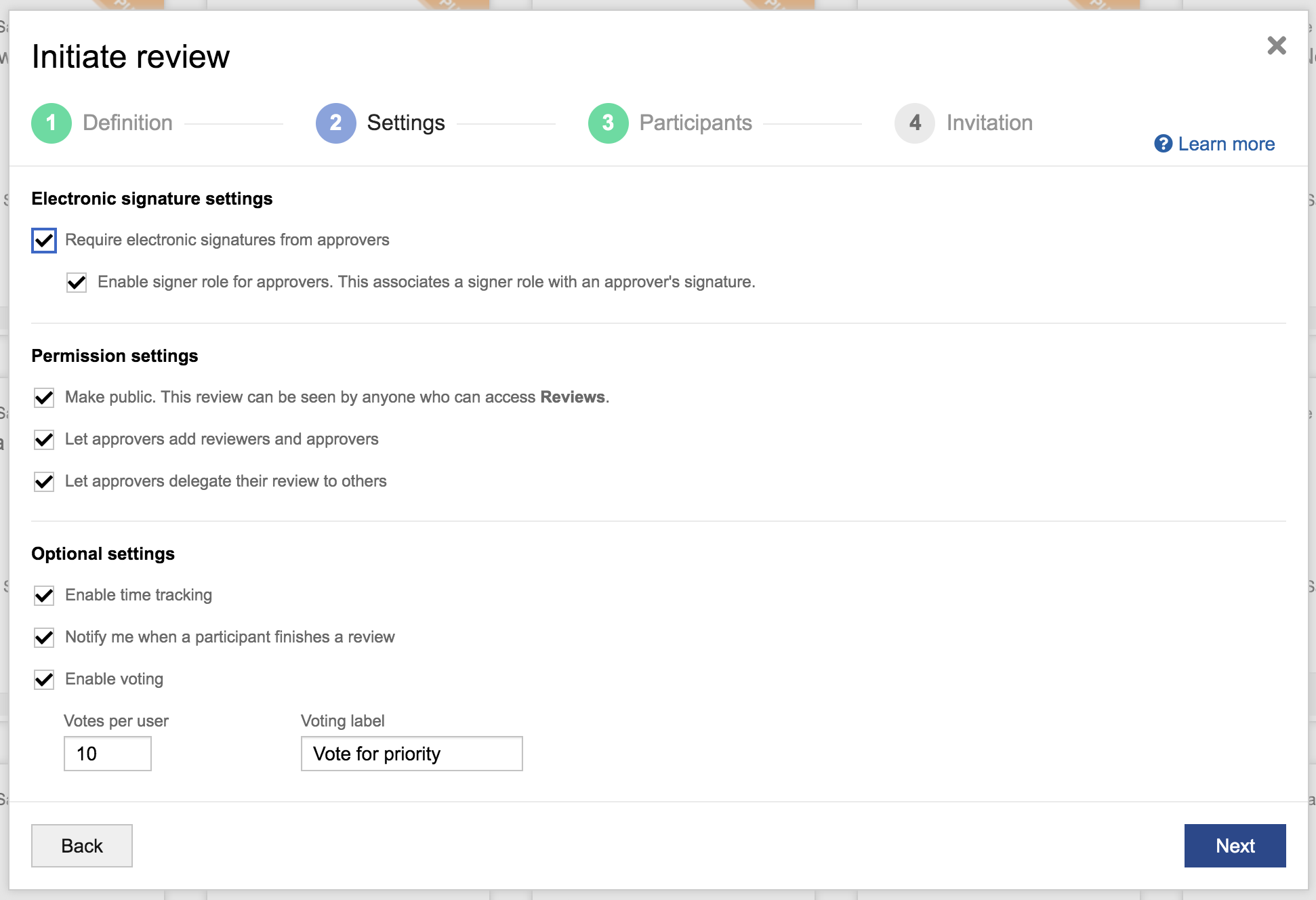
Streamline the review process with clearly identified participant roles and electronic signature settings.
These enhancements make the review process more straightforward and help customers meet compliance requirements for standards like FDA 21 CFR Part 11.
In the coming months, we’ll continue to invest in review and approval enhancements to improve collaboration and further address 21 CFR Part 11 compliance in Jama Connect Review Center.
Review Center Departs from Traditional, Document-Based Reviews
Traditional review processes often stifle collaboration and focus on a document-based approach, resulting in misalignment, long review cycles, versioning issues, and an abundance of unnecessary meetings.
By creating a centralized place to manage and collaborate on reviews, Jama Connect Review Center eliminates the need for lengthy, in-person meetings or back-and-forth emailing, making reviews more efficient and scalable.
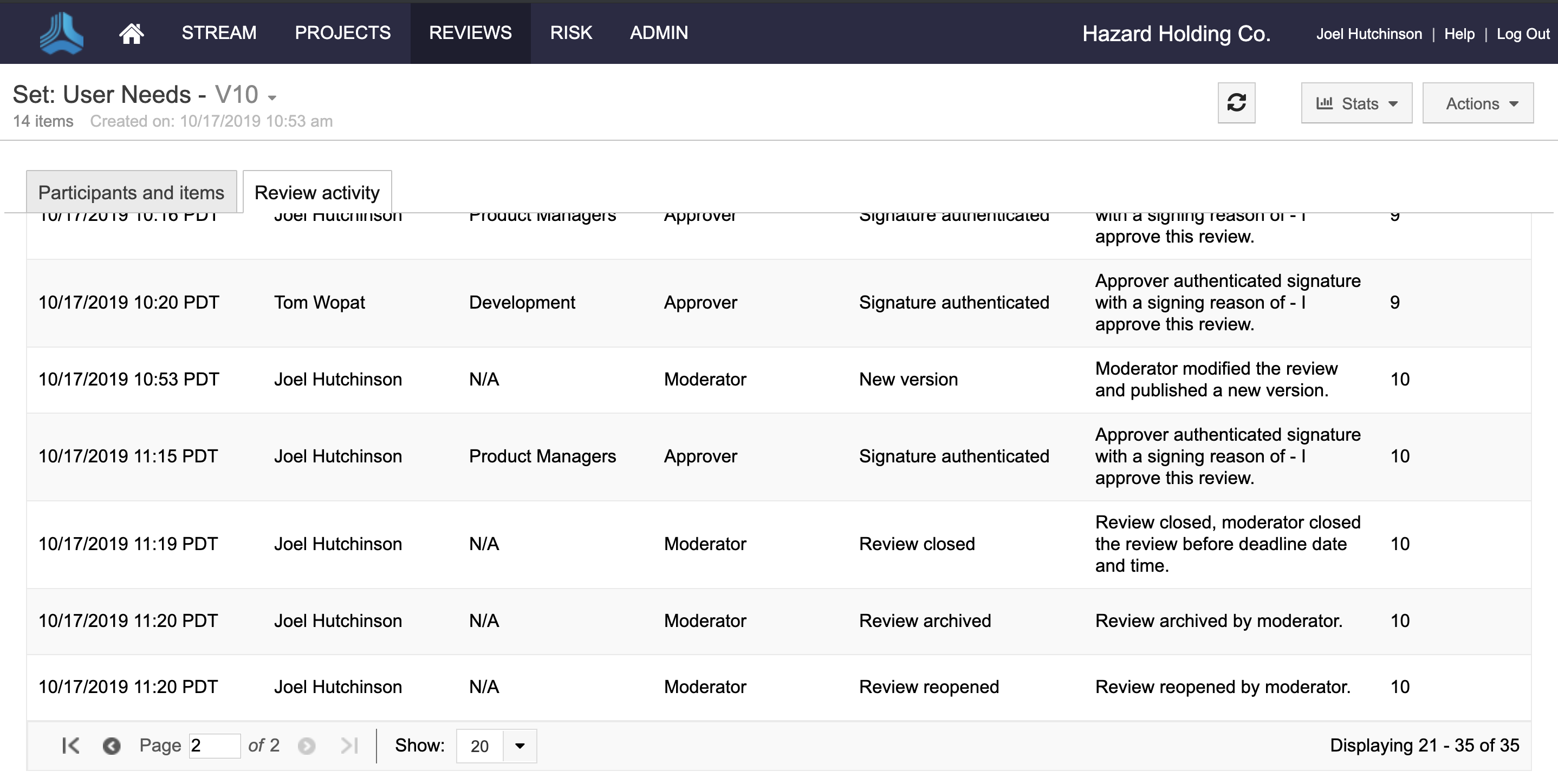
Gain visibility into all major review activities.
Michelle Seitz, Senior Business Analyst at MediSync, believes one of the most significant benefits of Jama Connect is the reduced time and effort it takes to complete review cycles using Review Center.
“My favorite part of Jama right now is when I get a collection of requirements to send out for a review,” Seitz says. “It is so much better with Jama. It’s the first time I’ve had a product that works as seamlessly as Jama does to produce a review and get feedback without having to do all the track changes and stuff that we used to have to do in Microsoft Word.”
And the results of the improved review process speak for themselves.
MediSync says that Jama Connect has saved the organization 80% of planning time that previously would have been wasted on meetings, sorting through versioned documents and emails, and consolidating feedback in review cycles.
Global healthcare leader Grifols says that Review Center has helped it shorten review cycles from three months to fewer than 30 days, while reducing budget overruns. They estimate savings of over 80 hours per project in medical device development thanks to Jama Connect Review Center.
As an organization, Jama Software is committed to improving stakeholder alignment, reducing lengthy review cycles, and easing the path to compliance by providing our customers with a modern approach to reviews and collaboration. And with Review Center’s new improvements and feature enhancements, we can confidently say that we are doing just that.
To learn more about best practices for moving through reviews quickly and seamlessly, download the “Jama Software Guide to Review Center Best Practices.”