Incorporating Risk Traceability into Manufacturing Production Software and Preparing for the Transition from CSV to CSA
Intro
Over the years, the burden of Computer Systems Validation (CSV) has resulted in medical device manufacturers avoiding implementation of automated manufacturing production systems or upgrading long-outdated versions of software. As part of the FDA’s ‘Case for Quality’ initiative in 2011 to study quality best practice in medical device manufacturing, the FDA found that the burden of compliance, such what is expected for Computer Systems Validation (CSV), deterred technology investments and as a result, inhibited quality best-practice.
As an outcome, the FDA is expected to release a draft guidance in 2022 that outlines their new approach of Computer Software Assurance (CSA) for Production and Quality System Software. The goal is to improve software quality by focusing on the software’s impact to patient safety, impact to product quality, and impact to quality system integrity. Using a risk-based approach, manufacturers can spend more time testing to ensure software meets its intended use, instead of ensuring test protocols and reports are fully scripted are error free.
As the key to right-sizing CSA activities is based on risk, managing risk, and risk traceability and incorporating is a key activity. And there’s no need to wait for the publication of the FDA CSA draft guidance, medical device manufacturers can incorporate these risk management and traceability practices now in their CSV activities.
Keep reading below for best practices on how to extend risk management and risk traceability from the medical device development to your manufacturing production software used to manufacture your devices.
Main Points for How
Consider the product risks and hazards
Even though we are discussing manufacturing production software and the software used on manufacturing equipment, the first step is to consider the product risks and hazards. Start with your master list of hazards, hazardous situations, and associated harms developed during medical device development and determine where and how failures in the manufacturing production software and production equipment can result in those risks and hazards. These are the areas to consider as the highest risks to control, mitigate, and validate.
Next, perform additional risk analysis from the lens of the environment and manufacturing personnel that will operate said equipment and software. It is important to also protect the environment and prevent injury and harm to those interacting with the equipment. You don’t want the unintended release of hazardous chemical reagents or equipment fires that could have been prevented with built-in high temperature shut-down feature.
RELATED: Managing Complexity in Systems Engineering for Product Development with Live Traceability
Create manufacturing production software intended uses and requirements
Just as one determines the intended use and design input requirements of a medical device, the same is to be done for the manufacturing production software and equipment. Incorporate the necessary features as manufacturing requirements (analogous to the design inputs of your medical device) that address the risks identified in the previous step.
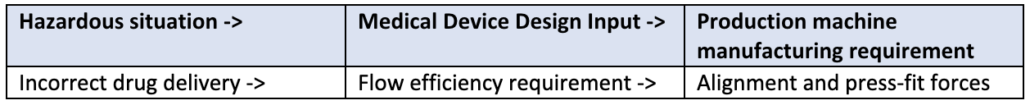
One example is based on risk of the product and demonstrates how risk traces from the development of the medical device. Consider the design of a semi-automated assembly machine for a drug-delivery device. The machine aligns and press-fits together two plastic injection-molded halves of the delivery sub-assembly. One of the main product associated risks is ensuring proper flow efficiency to ensure the proper amount of drug is delivered correctly and to the proper anatomical location.
In this case the risk traceability and translation to the assembly machine design inputs is:
RELATED: Application of Risk Analysis Techniques in Jama Connect® to Satisfy ISO 14971
Perform software testing and equipment validation based on risk
Once ready to test the production equipment software and perform equipment validation, incorporate, you guessed it, risk. All manufacturing requirements then need to be verified, however, scale the amount and type of testing for each manufacturing requirement based on the risk. Aspects of the software and equipment that are of high risk, such as preventing severe harm to operators, or the end users and patients of the medical device will have more testing and documentation than those of lower risk.
Document risk traceability of testing and equipment validation
Just as documenting risk traceability for medical device is important, it’s also important for manufacturing production software and equipment. Using a tool like Jama Connect® makes it easy to link your master list of hazards, hazardous situations, and harms to your manufacturing requirements for manufacturing production software and equipment. This increases consistency and efficiency in the risk analysis and prioritizing efforts on where it matters for patient and operator safety. The interface of Jama Connect® also makes the traceability and documentation of the associated testing and validation easy to visualize, alerting teams to gaps and incomplete testing.
Closing
Extending risk traceability from your medical device development to your manufacturing production software and equipment brings focus to what can impact patient safety and scales the testing and validation work appropriately. Incorporate risk and you’ll also be well on your way for the upcoming FDA transition from CSV to CSA.
- Practical Guide for Implementing Software Validation in Medical Devices: From FDA Guidance to Real-World Application – Part 2 - March 28, 2023
- Practical Guide for Implementing Software Validation in Medical Devices: From FDA Guidance to Real-World Application – Part I - March 7, 2023
- Common Pitfalls in Change Control Traceability and How to Compensate - January 17, 2023