Empowering Complex Medical Device and Life Sciences Development with Responsible AI and Machine Learning
Artificial intelligence (AI) and machine learning (ML) are transforming industries across the globe, and the medical device and life sciences sectors are no exception. Today, the development of complex medical devices is being accelerated and optimized with the integration of responsible AI. From enhanced product design to improved compliance and streamlined workflows, AI is allowing teams to accelerate their workflows.
This blog aims to explore the expected impact of AI on medical device development — including current industry trends, regulatory considerations, and how companies like Jama Software are empowering organizations to leverage AI responsibly.
Types of AI Medical Devices
AI medical devices are rapidly transforming healthcare by introducing advanced diagnostic, therapeutic, and monitoring capabilities. Among the 950 AI-powered medical devices currently identified, several core categories stand out:
- Diagnostic Tools: AI-driven diagnostic devices are designed to assist in identifying diseases and conditions with greater accuracy and efficiency. These include imaging systems equipped with machine learning algorithms to detect abnormalities in X-rays, MRIs, and CT scans, as well as AI software for analyzing pathology reports or genetic data. Such tools are especially beneficial in early disease detection, reducing diagnostic errors, and improving patient outcomes.
- Monitoring Devices: Another significant category is AI-enabled monitoring devices. These systems continuously track patient vitals, such as heart rate, blood pressure, glucose levels, or oxygen saturation, and can alert healthcare providers to early warning signs of complications. Wearable sensors and remote patient monitoring platforms powered by AI are playing a crucial role in providing real-time health insights, especially for chronic disease management.
- Therapeutic Systems: AI-powered therapeutic devices support or enhance treatment strategies. Examples include robotic surgery platforms with AI for precise surgical execution, as well as AI software that tailors treatment plans using patient-specific data. These systems are helping doctors optimize therapies and deliver more effective and personalized care.
- Decision Support Applications: AI-based decision support tools are designed to assist clinicians in making informed decisions. These devices analyze vast amounts of medical data to provide evidence-based recommendations or predict potential complications. They are widely used to guide treatment strategies and manage complex conditions with a data-driven approach.
By focusing on these categories, it becomes clear that AI medical devices are not only advancing the standard of care but also addressing critical gaps in healthcare delivery through innovative applications of artificial intelligence.
Medical device manufacturers are leveraging AI and ML in several groundbreaking ways. The U.S. Food and Drug Administration (FDA) has already approved over 950 AI-enabled medical devices as of mid-2024. This reflects a dramatic rise over the past decade and highlights the growing adoption of AI technologies. Here are key areas where AI is making a difference in medical device development:
Current Regulations for AI and ML in Medical Device Development
While the potential of AI is evident the successful adoption of these technologies requires adherence to evolving regulatory frameworks. The FDA has been actively addressing the need for guidelines in this space, ensuring a balance between innovation and safety.
FDA Guidance and Regulations
The FDA oversees AI-enabled devices through various pre-market pathways, including:
- Premarket Notification (510(k))
- De Novo Classification
- Premarket Approval (PMA)
To accommodate the complexities associated with adaptive AI/ML-enabled devices, the FDA has released multiple resources, such as the 2024 Artificial Intelligence and Machine Learning Action Plan, which outlines the agency’s vision for regulatory oversight. Key highlights include:
- Development of Good Machine Learning Practices (GMLP).
- Introduction of frameworks for Predetermined Change Control Plans, allowing for post-market updates to AI systems without requiring new approvals.
- Enhanced transparency for AI-powered medical devices.
New FDA Guidance: To learn more about recent guidance provided by the FDA, read these two articles:
- Marketing Submission Recommendations for a Predetermined Change Control Plan for Artificial Intelligence-Enabled Device Software Functions
- Artificial Intelligence-Enabled Device Software Functions: Lifecycle Management and Marketing Submission Recommendations
Global Considerations
Beyond the U.S., regulatory developments in Europe, including the EU AI Act and MDR compliance, are impacting how organizations worldwide approach AI implementation in medical devices.
Staying compliant demands robust traceability, stringent risk management protocols, and adherence to data security standards.
RELATED: Buyer’s Guide: Selecting a Requirements Management and Traceability Solution for Medical Device & Life Sciences
Trends and Insights for AI-Powered Medical Device Development
The trajectory of AI in medical device development is poised to grow exponentially, driven by several key trends:
Shift to Software-Defined Devices
Medical devices are increasingly becoming software-centric. Traditional hardware is giving way to platforms that can be continuously enhanced through software updates.
Integration of Generative AI
Generative AI algorithms are now being used by medical device manufacturers to create simulations, generate testing data, and optimize device designs. Companies like NVIDIA are spearheading this trend with platforms supporting robotics and digital surgery.
Rise of Collaboration and Ecosystem Growth
Partnerships between MedTech companies, academic institutions, and tech giants are fueling innovation. Startups like Moon Surgical and Aidoc demonstrate how collaborations can lead to cutting-edge AI implementations in laparoscopy and diagnostic tools.
Broader Applications Beyond Radiology
While radiology remains at the forefront, AI is expanding rapidly into fields like:
- Neurology: Detecting brain activity anomalies.
- Cardiology: Spotting arrhythmias through AI-powered stethoscopes.
- Oncology: AI-enabled tools for precision cancer diagnosis.
RELATED: Industry-leading Practices Modernize Legacy Public Health Software System, a Deloitte Customer Story
Jama Software’s AI Initiatives for Medical Device Development
With the rise of AI-enabled medical devices, Jama Software is focused on how organizations can incorporate AI applications in their product development processes to improve quality and accelerate development. Here are a few example use cases Jama Connect® is focused on:
Jama Connect Advisor™
Jama Connect Advisor analyzes your product requirements against industry standards such as INCOSE Rules and EARS Notation, then recommends improvements. This fast, accurate analysis and advice helps your requirements align with engineering-focused natural language and best practices.
Benefits of using Jama Connect Advisor:
- Improves the quality, accuracy, and usability of your requirements across your organization.
- Assists development teams in standardizing the process and language of requirement authoring.
- Saves time for authoring, reviewing, and updating requirement statements.
- Helps deliver programs and projects on time and on budget.
- Reduces the risk of late-stage errors.
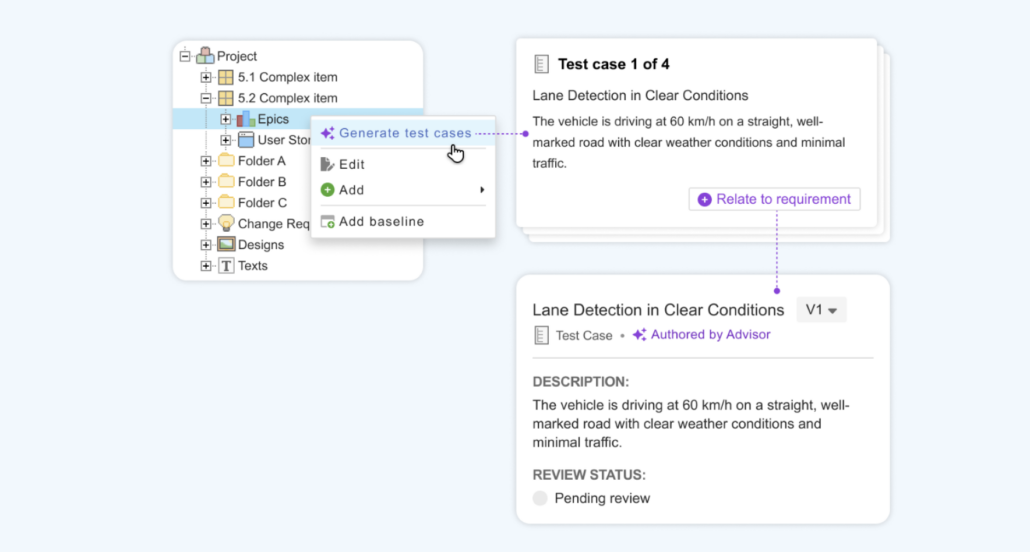
Test Case Intelligence
Streamline verification and accelerate to market with AI-generated test cases derived from requirements.
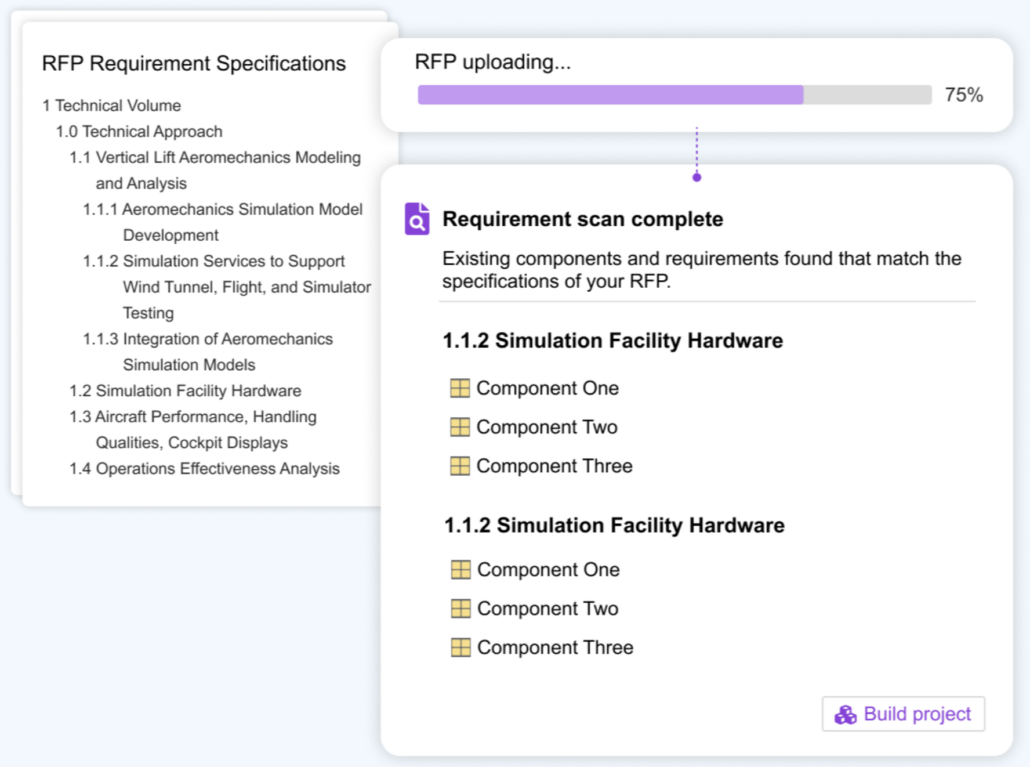
Intelligent PDF Parsing
Quickly parse PDFs and Office files, match to existing IP, or generate new requirements for review and collaboration. AI enhances reuse, speeds RFPs, streamlines supply chain collaboration, and boosts product quality.
Learn more about Jama Software’s AI initiatives by visiting: https://labs.jamasoftware.com/
Transforming the Future with Responsible AI
Integration of AI and ML is already accelerating and changing medical device development. From enabling groundbreaking technologies to revolutionizing patient care, AI is laying the foundation for the medical devices of tomorrow. However, success in this domain requires balancing innovation with compliance, backed by robust tools that alleviate complexity.
Jama Software’s AI solutions empower organizations to tackle these challenges head-on, reducing risks, streamlining workflows, and ensuring quality at every step of medical device development.
Want to see how AI can streamline your product development?
Book your free demo today and experience the power of AI in revolutionizing requirements management for medical devices.
- Empowering Complex Medical Device and Life Sciences Development with Responsible AI and Machine Learning - June 20, 2025
- [Webinar Recap] Systems Engineering MedTech Challenges - February 18, 2025
- Shaping the Future of MedTech: Insights from Industry Leaders on AI, Innovation, and Regulatory Challenges - December 17, 2024