Solution components in the Jama Connect for Medical Device Development solution help teams reduce time-to-value, provide guidance around customer-specific needs, and drive adoption.
We wanted the solution to offer a collection of training and documentation components that aligns to industry regulations so product development teams could get ramped up quickly.
The result: an out-of-the-box configuration of Jama Connect, an accompanying Procedure Guide, and Jama Professional Services. It’s a solution designed to reduce time-to-value, account for and provide tailored guidance around customer-specific needs, and drive adoption through tailored training and on-going support. Let’s take a look at the components and see how they help achieve those goals.
Note: Now that our medical device blog series is concluded, you can go back and read the series intro, Part I, and Part III.
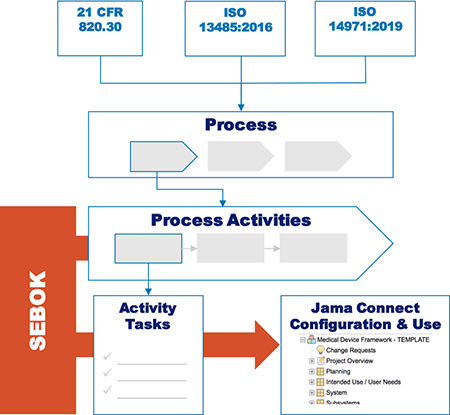
Procedure and Configuration guide: Jama Connect, clearly aligned with regulations.
The Jama Connect for Medical Device Development Procedure and Configuration Guide provides detailed alignment between these regulations and standards and the out of the box configuration and recommended use of Jama Connect:
- ISO 13485:2016
- ISO 14971:2019
- 21 CFR 820.30
The scope and processes described in the guide are clearly identified and justified.
An important point to note: we identified the Jama Connect capabilities that align with the most relevant design control requirements needs. Then we targeted those needs, and optimized Jama Connect to meet them exactly.
The regulations and standards establish the design control requirements and provide some guidance, but they are not prescriptive in terms of tools or techniques. The procedure and configuration guide explains in detail how to bring in best practices from systems thinking, supported by use of Jama Connect, while still complying with design control requirements. .
Teams building complex medical devices will benefit from this systems-thinking approach and the way the Procedure and Configuration Guide builds these layers into the configuration. The principles and guidance provided by the Systems Engineering Body of Knowledge (SEBoK) and the recommended use of Jama Connect will bring clarity around product definition and verification activities.
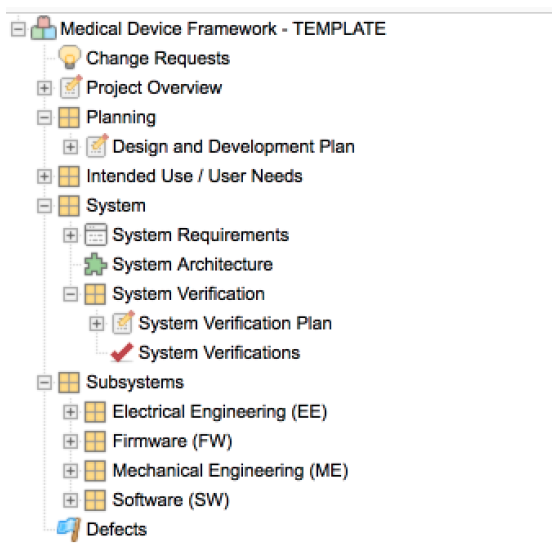
Out-of-the-box-configuration: Start with standardized guidance and build from there.
The out-of-the-box configuration provides a recommended project template that allows customers to get up and running quickly. It also serves as a starting point for customer-specific discovery and configuration. Each aspect of the configuration aligns with the applicable regulations and standard through detailed process activities and tasks described in the procedure guide
The configuration also provides Export Templates you can use to generate documentation from Jama Connect for transport into your Quality Management System (QMS), typically used to house all Design History Files for sign-off and audit.
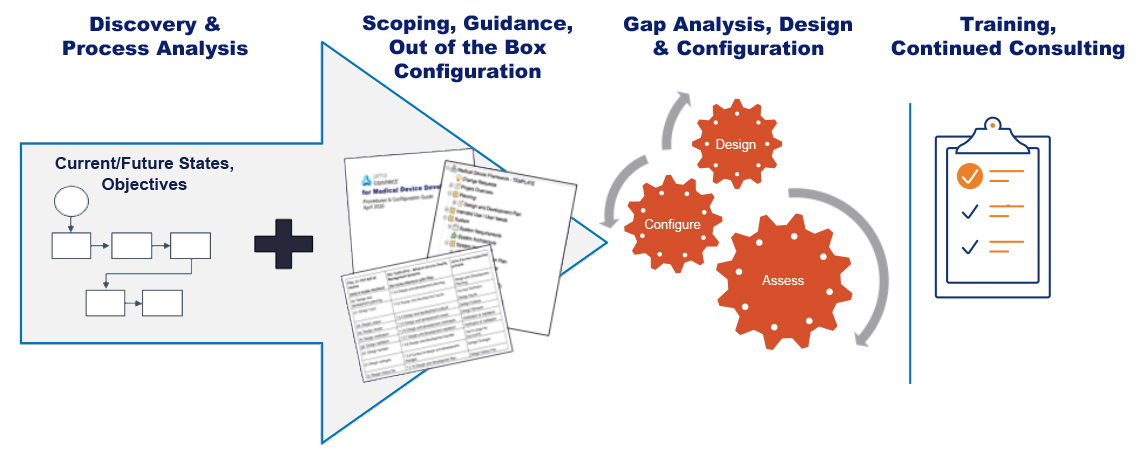
On-site consulting and training services to align your teams quickly.
Jama Software’s Professional Services team works to help customers reach several goals:
- Build adoption, decrease time-to-value, and maintain ease of use.
- Focus on process alignment, people readiness, and best practices for configuration and use.
- Take advantage of the recommendations and guidance described in the Procedure and Configuration Guide and built into the out of the box configuration.
- Provide end-user training tailored to the customer’s use and configuration of Jama Connect .
Taken as a whole, the Jama Connect for Medical Device Development solution provides the guidance, justification and a starting framework that not only answers “what” Medical Device manufacturers should do when deploying Jama Connect, but also “how” and “why” they should do it.
In upcoming blogs in this series, we’ll explore specific aspects of the Procedure and Configuration Guide and the out of the box configuration of Jama Connect. We’ll also look at how the solution components described in this blog are applied in practice.
Download a Solution Brief for an overview that explains all the ways Jama Connect for Medical Device Development can help your teams manage shifting complexities and maintain product quality and safety.
- Jama Connect® Features in Five: Reuse & Sync - November 3, 2023
- Part V: Using the Trace as a Way to Work - June 25, 2020
- Part IV: Connecting Design Controls, Including Design Inputs, Design Outputs and Verifications - June 18, 2020