Jama Software Provides a Single, All-in-One Solution for Requirements, Risk Management, and Validation
Organizing requirements, managing risk, and ensuring validation are complex processes on their own. For teams in the medical device industry, these tasks are even more challenging due to stringent regulatory standards and the critical nature of the products themselves. Jama Connect®, a robust software tool designed specifically for such pressures, offers a seamless, all-in-one solution.
What to Look for In a Requirements Management Tool
At the 2024 INCOSE Healthcare Conference, one of the presenters delivered an insightful presentation highlighting the essential features their organization identified as necessary in a requirements management solution to effectively support complex risk management procedures.
This blog post provides a detailed breakdown of how Jama Connect is specifically designed to address each of these critical areas discussed during the presentation.
End-to-End Traceability for Risk Analysis and Validation
Why It’s Important: Without clear traceability, unverified requirements or overlooked risks can result in delays, missed compliance, or worse, product failure.
How Jama Connect Helps:
- Jama Connect provides traceability across all items in the development process, linking risk analysis elements to requirements, mitigations, and verification items.
- Suspect triggers and impact analysis. Any upstream change triggers “suspect links,” highlighting potential downstream impacts, so no critical changes are overlooked. Impact analysis features allow for a proactive approach to understanding the scope of a change BEFORE it occurs.
- Configurable views and export templates ensure efficient reporting to compliance agencies, showing validation evidence clearly and concisely.
Example: Teams can easily export views with verification columns from Jama Connect into Word or Excel for seamless external reporting.
Integrated FMEA Capabilities
Why It’s Important: Proper Failure Mode and Effects Analysis (FMEA) evaluates risks effectively and shows compliance with ISO 14971 for medical devices.
How Jama Connect Helps:
- Full support for FMEA tracking, including automated RPN (Risk Priority Number) calculations and risk matrices to simplify decision-making.
- Pre-configured medical device frameworks allow quicker adoption while still being customizable to match specific workflows.
- Tools like exportable templates make it easy to share mitigation strategies with stakeholders.
For complex risk calculations, teams can leverage Jama Connect Interchange™, ensuring that even intricate needs are met reliably.
- Additional Resource: Watch this video to see how Jama Connect facilitates FMEA.
- Additional Resource: Read about FMEA in our Requirements Guide
RELATED: Accelerate the Development of Safe and Effective Medical Devices & Life Science Products with Jama Connect
Standard Frameworks for Risk Management
Why It’s Important: Medical device standards aren’t optional; they’re essential for safety and compliance.
How Jama Connect Helps:
- Enables compliance with key standards like ISO 14971, integrating these directly into risk frameworks along with thorough hazard documentation.
- With pre-configured templates, teams can efficiently manage risk assessments without manually recreating workflows.
For development teams, having standards built into the process reduces human error and increases efficiency.
Version Control and Change Management
Why It’s Important: Medical devices often undergo project iterations where changes can impact earlier decisions. Keeping track of these is vital.
How Jama Connect Helps:
- Change management item types can trace changes back to risk assessments, ensuring traceability.
- Built-in features allow versioned tracking for every modification, maintaining compliance and detailed documentation.
- Connections between mitigations, risks, and validations ensure transparency in every decision made.
Change management is a core feature that proves invaluable when audits require detailed project histories.
Usability and Configuration
Why It’s Important: Even the most advanced tool is counterproductive if it’s hard to use or implement.
How Jama Connect Helps:
- Jama Connect is celebrated on the G2 Grid as the highest-ranked requirements management tool, with accolades for its ease of use.
- Supports seamless data imports from applications like Microsoft Excel or Word, using reusable import wizards for faster and repeatable imports.
- Pre-configured framework for Medical Device & Life Sciences, reduces time-to-value, allowing teams to get up and running quickly.
- SOC2 Compliance, combined with the robust capabilities of a Validated Cloud and Validation Kit, provides systems engineers with effective solutions to meet stringent security and regulatory compliance requirements.
Additionally, the focus on user experience allows for a fast time to value across project teams.
Flexible Configurations for Different Workflows
Why It’s Important: Medical device companies don’t all operate under the same workflow, and tools must accommodate that diversity.
How Jama Connect Helps:
- Pre-configured frameworks for compliance streamline setup time while allowing for configuration to suit unique organizational processes.
- Provides users with the Traceability Information Model, visualizing traceability chains to ensure nothing falls through the cracks.
- From aligning with new SOPs to creating testing and validation workflows, customization ensures this tool adapts as projects evolve.
Scalable Solution for Large Projects
Why It’s Important: Medical device development often spans multiple teams and product lines, increasing complexity.
How Jama Connect Helps:
- Provides for the ability to break large projects down into smaller, manageable subcomponents while maintaining traceability across product lines.
- Supports cross-project traceability and reusability, which is ideal for future scaling.
- This modular approach makes it easy for businesses to simplify operations without losing sight of critical compliance elements.
RELATED: Align Safety Hazards, Security Threat Analysis, Risk Assessments, and Functional Safety Directly into Your Engineering Workstream
Tool Configuration for Effective Risk Management
Risk management and requirements traceability go hand-in-hand, forming a critical component of any successful project. A well-configured tool should seamlessly align with your existing processes while offering the flexibility to adapt to your specific needs.
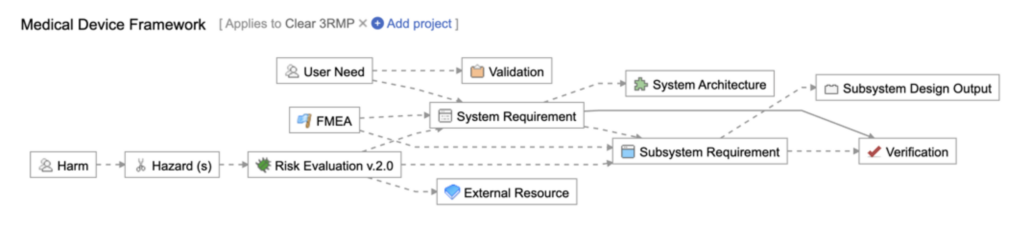
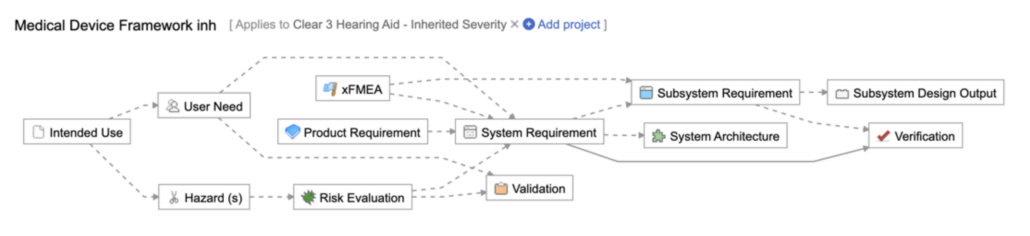
To illustrate, here are three examples of how Jama Connect’s Traceability Information Model can be configured to enhance and support a comprehensive risk management process. These configurations are designed to streamline workflows, improve traceability, and ensure better alignment with your risk management objectives.
Example 1 (from Jama Connect’s Medical Device Framework)
Example 2 (from Jama Connect’s Medical Device Framework)
Example 3: Customized Workflow
Conclusion:
Jama Connect is a purpose-built solution designed to meet the complex and stringent requirements of the medical device industry. Offering robust capabilities like end-to-end traceability — from risk analysis through to verification — it ensures seamless oversight across the entire development lifecycle. With capabilities, such as FMEA and alignment with critical regulatory standards for the medical device industry, Jama Connect simplifies compliance and elevates requirements management to the next level.
With award winning usability, Jama Connect features an intuitive interface, customizable configurations, and powerful change management capabilities. These features enable teams to work efficiently while staying aligned with regulatory needs. By fostering real-time collaboration and bridging the gaps between stakeholders, Jama Connect empowers organizations to accelerate product development without compromising on safety or quality.