Jama Software & Abbott Laboratories
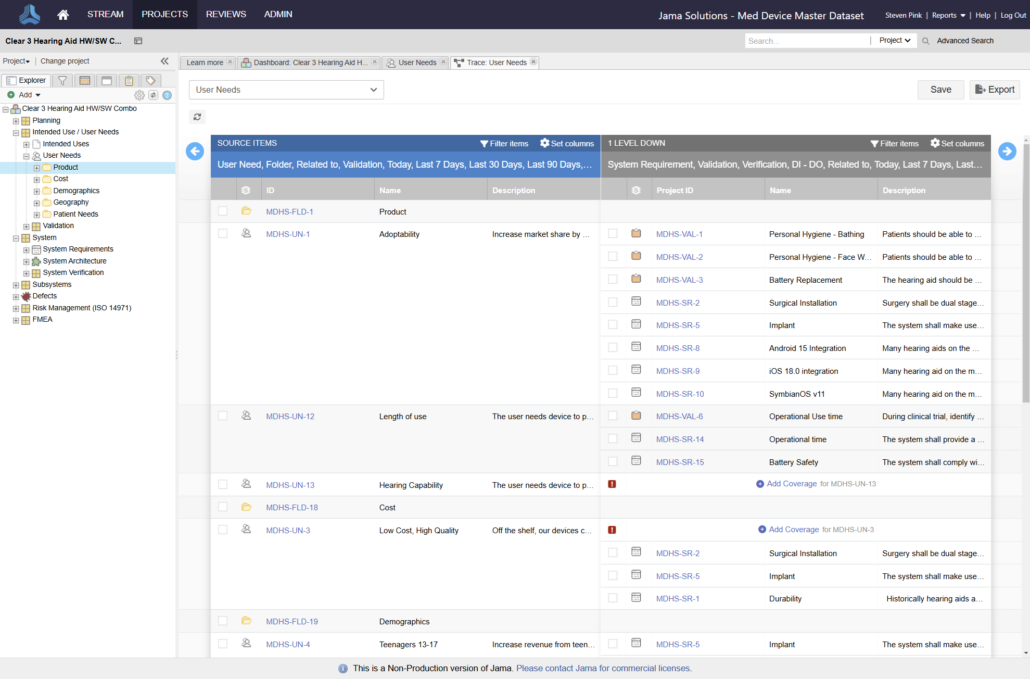
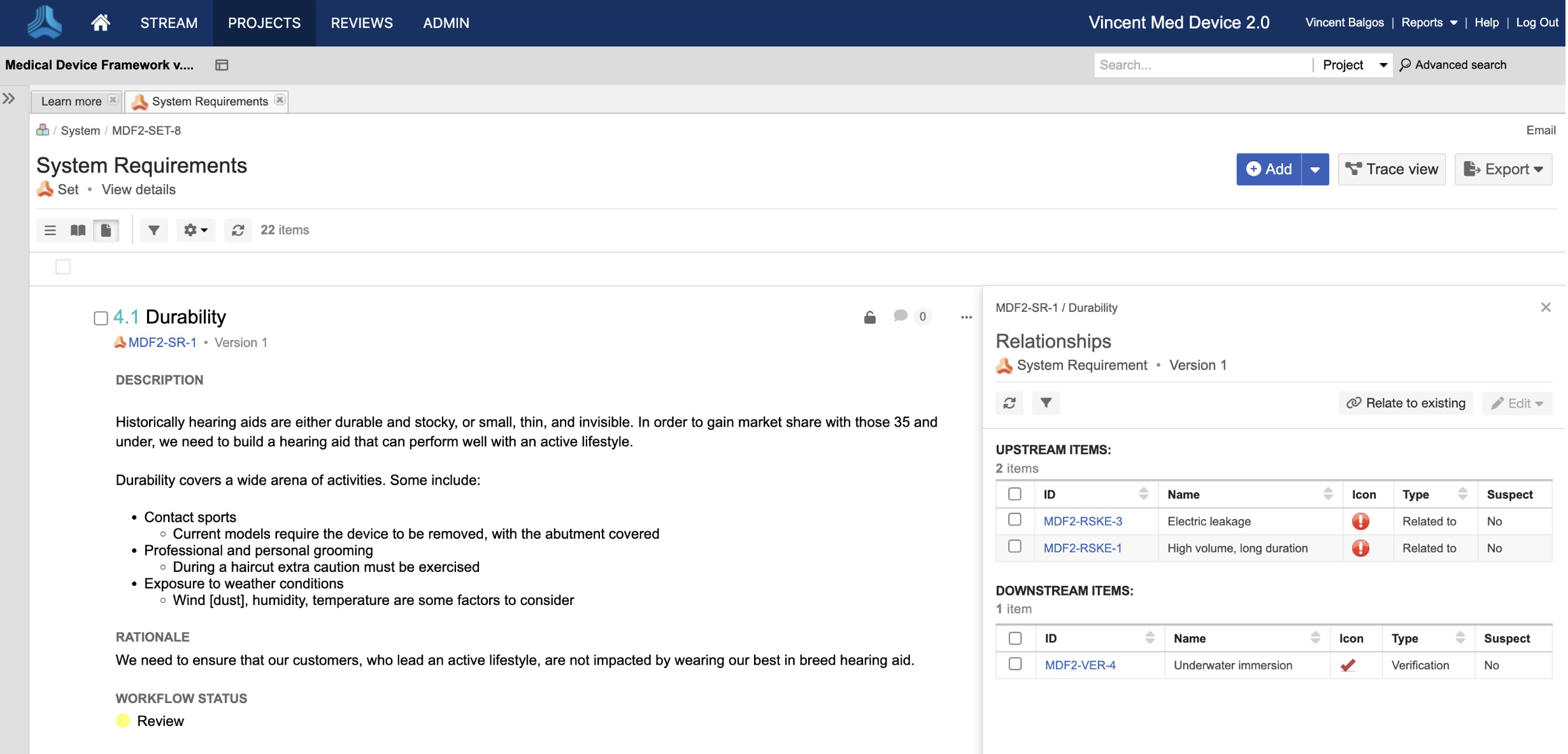
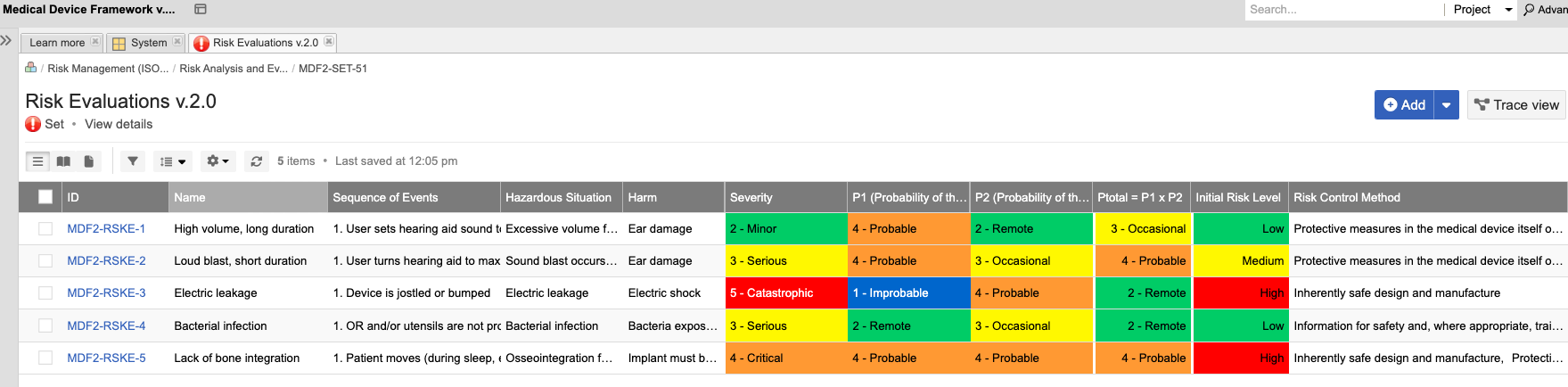
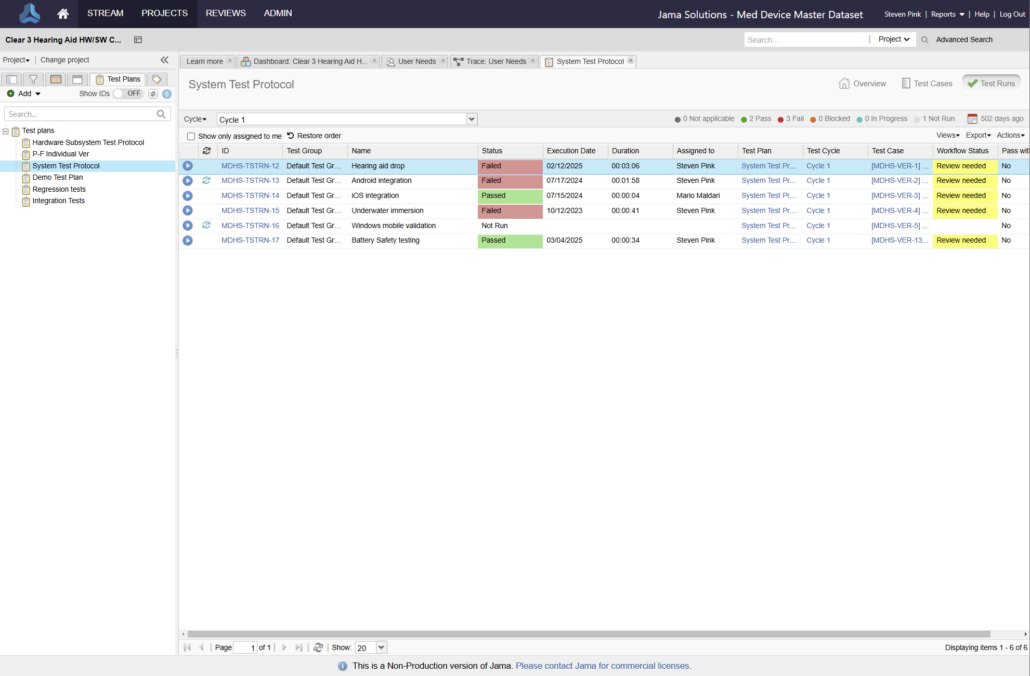
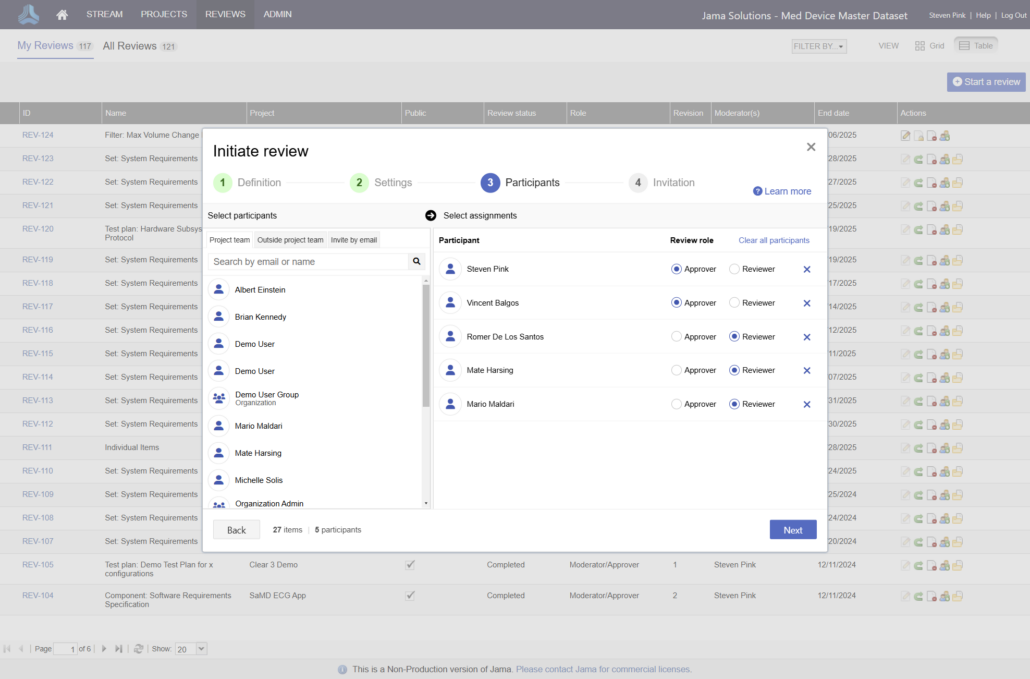
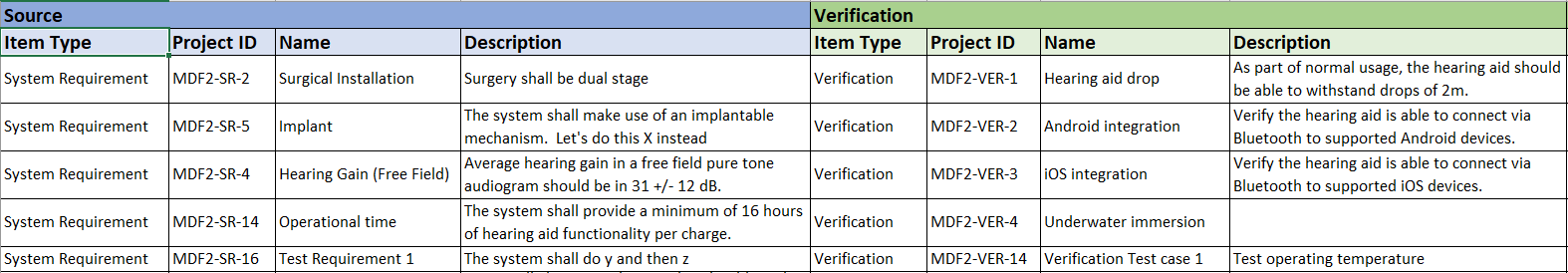
Jama Connect® is the platform of choice for 8 of the top 10 global medical device companies and numerous divisions across Abbott.
Join the growing number of Abbott businesses utilizing Jama Connect to modernize their design controls and risk management processes!