Accelerate innovation & streamline your processes in medical device development
On every critical dimension, Jama Software is the Requirements Management leader: market share, user reviews, user community, usability, adoption, client success, scale, advanced capabilities, product investment, and more. We have grown through word of mouth for a reason – our users love us, bring us to their new companies, and spread the word. We’d love for you to join the Jama Software family!
Reduce Risk
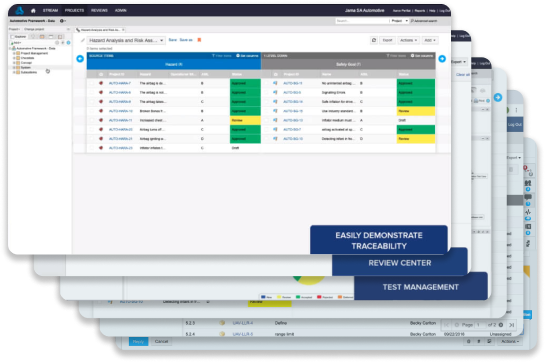
Hazard & risk assessment decreases risk of rework and recalls, and increases product quality
Develop Faster
Configuration and export templates & frameworks reduce time to market
Collaborate Easier
Stakeholders can view/comment on open decisions, issues, and questions in real-time
Iterate with Agility
Review center, reuse, and baseline management allow teams to move quickly and stay on track.
Stay Compliant
Standard frameworks aligned to industry regulations ISO 13485, ISO 14971, IEC 62304, 21 CFR 820.30, CFR Part 11, AAMI TIR45
Align Teams
Live Traceability™ aligns teams, processes, and tools across the development cycle.
The G2 Grid® #1 platform for Requirements Management + in-house industry experts = development success
Jama Connect for Medical Device Development includes:
Unlimited licenses for Stakeholders and Reviewers24×7 critical-issue support
Standard industry frameworksFunctional Safety Kit for Jama Connect
Expert training & consulting designed for medical manufacturing
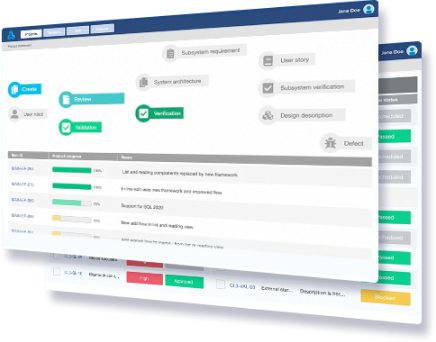
Live Traceability
Reviews & Approvals
Test & Quality Management
Risk & Hazard Analysis
Reuse & Baseline Management
Real-Time Collaboration
Take your products to market faster with Jama Connect®
Take a look at our award-winning platform today!